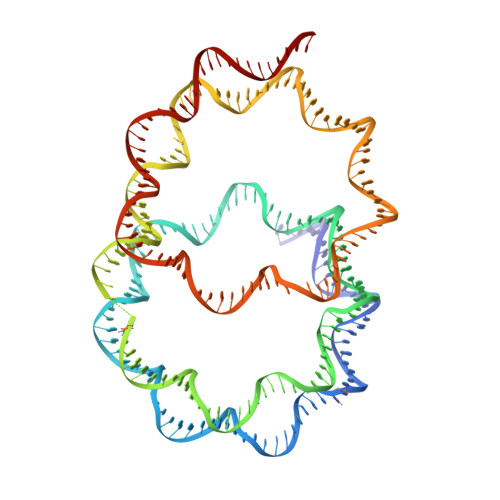
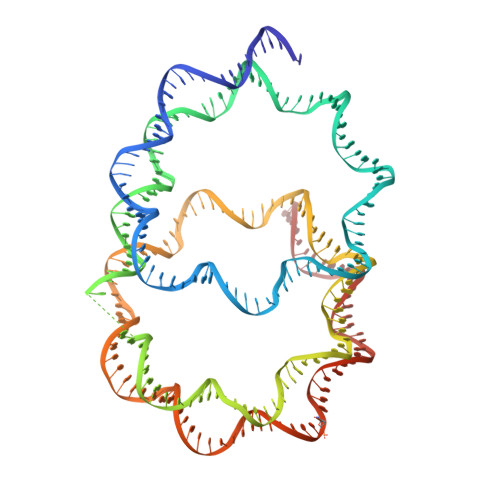
Crystal structure of the overlapping dinucleosome composed of hexasome and octasome
Kato, D., Osakabe, A., Arimura, Y., Mizukami, Y., Horikoshi, N., Saikusa, K., Akashi, S., Nishimura, Y., Park, S.Y., Nogami, J., Maehara, K., Ohkawa, Y., Matsumoto, A., Kono, H., Inoue, R., Sugiyama, M., Kurumizaka, H.(2017) Science 356: 205-208
- PubMed: 28408607
- DOI: https://doi.org/10.1126/science.aak9867
- Primary Citation of Related Structures:
5GSE - PubMed Abstract:
Nucleosomes are dynamic entities that are repositioned along DNA by chromatin remodeling processes. A nucleosome repositioned by the switch-sucrose nonfermentable (SWI/SNF) remodeler collides with a neighbor and forms the intermediate "overlapping dinucleosome." Here, we report the crystal structure of the overlapping dinucleosome, in which two nucleosomes are associated, at 3.14-angstrom resolution. In the overlapping dinucleosome structure, the unusual "hexasome" nucleosome, composed of the histone hexamer lacking one H2A-H2B dimer from the conventional histone octamer, contacts the canonical "octasome" nucleosome, and they intimately associate. Consequently, about 250 base pairs of DNA are left-handedly wrapped in three turns, without a linker DNA segment between the hexasome and octasome moieties. The overlapping dinucleosome structure may provide important information to understand how nucleosome repositioning occurs during the chromatin remodeling process.
Organizational Affiliation:
Laboratory of Structural Biology, Graduate School of Advanced Science and Engineering, Waseda University, 2-2 Wakamatsu-cho, Shinjuku-ku, Tokyo 162-8480, Japan.