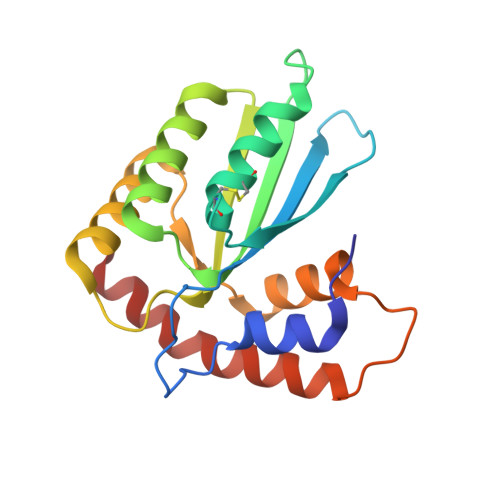
Understanding the Structure, Multimerization, Subcellular Localization and mC Selectivity of a Genomic Mutator and Anti-HIV Factor APOBEC3H.
Ito, F., Yang, H., Xiao, X., Li, S.X., Wolfe, A., Zirkle, B., Arutiunian, V., Chen, X.S.(2018) Sci Rep 8: 3763-3763
- PubMed: 29491387
- DOI: https://doi.org/10.1038/s41598-018-21955-0
- Primary Citation of Related Structures:
5W45 - PubMed Abstract:
APOBEC3H (A3H) is a member of the APOBEC3 subfamily of DNA cytosine deaminases that are important for innate immune defense and have been implicated in cancer biogenesis. To understand the structural basis for A3H biochemical function, we determined a high-resolution structure of human A3H and performed extensive biochemical analysis. The 2.49 Å crystal structure reveals a uniquely long C-terminal helix 6 (h6), a disrupted β5 strand of the canonical five-stranded β-sheet core, and a long loop 1 around the Zn-active center. Mutation of a loop 7 residue, W115, disrupted the RNA-mediated dimerization of A3H yielding an RNA-free monomeric form that still possessed nucleic acid binding and deaminase activity. A3H expressed in HEK293T cells showed RNA dependent HMW complex formation and RNase A-dependent deaminase activity. A3H has a highly positively charged surface surrounding the Zn-active center, and multiple positively charged residues within this charged surface play an important role in the RNA-mediated HMW formation and deaminase inhibition. Furthermore, these positively charged residues affect subcellular localization of A3H between the nucleus and cytosol. Finally, we have identified multiple residues of loop 1 and 7 that contribute to the overall deaminase activity and the methylcytosine selectivity.
Organizational Affiliation:
Molecular and Computational Biology, Departments of Biological Sciences and Chemistry, University of Southern California, Los Angeles, CA, 90089, USA.