Hydroxyethyl cellulose matrix applied to serial crystallography
Sugahara, M., Nakane, T., Masuda, T., Suzuki, M., Inoue, S., Song, C., Tanaka, R., Nakatsu, T., Mizohata, E., Yumoto, F., Tono, K., Joti, Y., Kameshima, T., Hatsui, T., Yabashi, M., Nureki, O., Numata, K., Nango, E., Iwata, S.(2017) Sci Rep 7: 703-703
- PubMed: 28386083
- DOI: https://doi.org/10.1038/s41598-017-00761-0
- Primary Citation of Related Structures:
5WR8, 5WR9, 5WRA, 5WRB, 5WRC - PubMed Abstract:
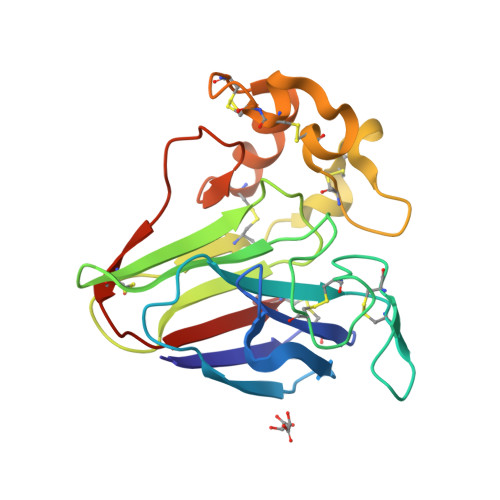
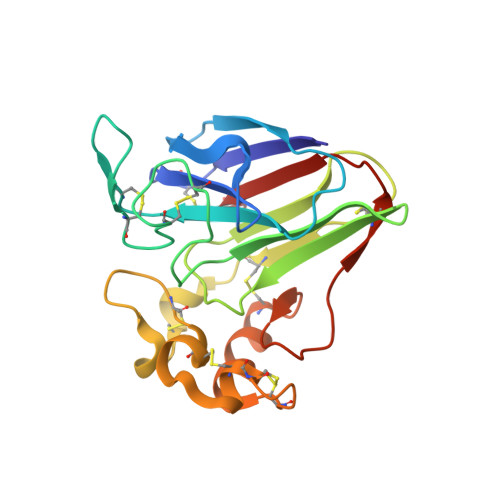
Serial femtosecond crystallography (SFX) allows structures of proteins to be determined at room temperature with minimal radiation damage. A highly viscous matrix acts as a crystal carrier for serial sample loading at a low flow rate that enables the determination of the structure, while requiring consumption of less than 1 mg of the sample. However, a reliable and versatile carrier matrix for a wide variety of protein samples is still elusive. Here we introduce a hydroxyethyl cellulose-matrix carrier, to determine the structure of three proteins. The de novo structure determination of proteinase K from single-wavelength anomalous diffraction (SAD) by utilizing the anomalous signal of the praseodymium atom was demonstrated using 3,000 diffraction images.
Organizational Affiliation:
RIKEN SPring-8 Center, 1-1-1 Kouto, Sayo-cho, Sayo-gun, Hyogo, 679-5148, Japan. msuga@spring8.or.jp.