6DFG
BG505 MD39 SOSIP trimer in complex with mature BG18 fragment antigen binding
- PDB DOI: https://doi.org/10.2210/pdb6DFG/pdb
- EM Map EMD-7875: EMDB EMDataResource
- Classification: VIRAL PROTEIN/IMMUNE SYSTEM
- Organism(s): Human immunodeficiency virus 1, Homo sapiens
- Expression System: Homo sapiens
- Mutation(s): No
- Deposited: 2018-05-14 Released: 2019-11-06
- Funding Organization(s): National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID), Bill & Melinda Gates Foundation
Experimental Data Snapshot
- Method: ELECTRON MICROSCOPY
- Resolution: 4.42 Å
- Aggregation State: PARTICLE
- Reconstruction Method: SINGLE PARTICLE
wwPDB Validation 3D Report Full Report
This is version 2.0 of the entry. See complete history.
Macromolecules
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Envelope glycoprotein gp160 | A, E [auth C], I [auth D] | 476 | Human immunodeficiency virus 1 | Mutation(s): 0 Gene Names: env | 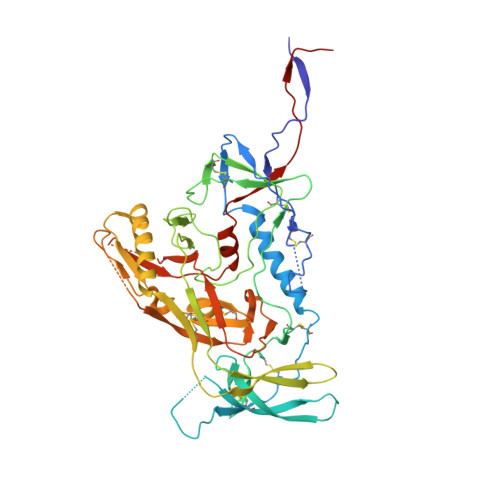 |
UniProt | |||||
Find proteins for Q2N0S6 (Human immunodeficiency virus 1) Explore Q2N0S6 Go to UniProtKB: Q2N0S6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q2N0S6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Envelope glycoprotein gp160 | B, F [auth E], J [auth F] | 162 | Human immunodeficiency virus 1 | Mutation(s): 0 Gene Names: env | 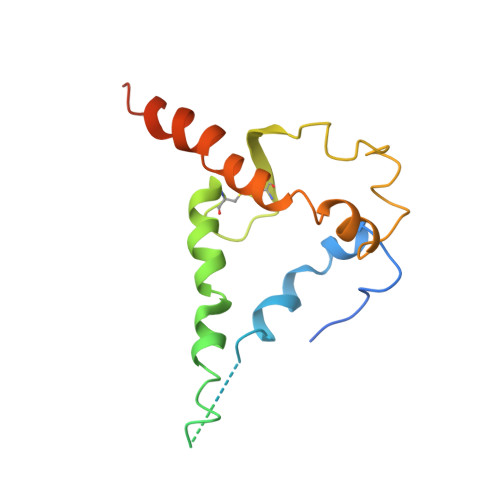 |
UniProt | |||||
Find proteins for Q2N0S8 (Human immunodeficiency virus 1) Explore Q2N0S8 Go to UniProtKB: Q2N0S8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q2N0S8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| mature BG18 fragment antigen binding heavy chain | C [auth H], G, K [auth I] | 233 | Homo sapiens | Mutation(s): 0 | 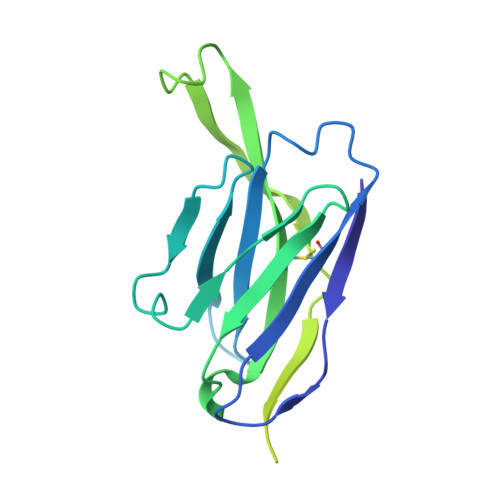 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| mature BG18 fragment antigen binding light chain | D [auth L], H [auth J], L [auth K] | 214 | Homo sapiens | Mutation(s): 0 | 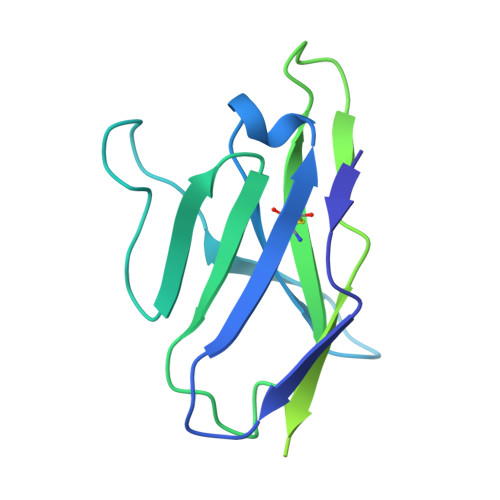 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Oligosaccharides
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-3)-beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | GA [auth g], M, W | 4 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G81315DD GlyCosmos: G81315DD GlyGen: G81315DD | |||||
Entity ID: 6 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | AA [auth a], DA [auth d], FA [auth f], HA [auth h], IA [auth i], AA [auth a], DA [auth d], FA [auth f], HA [auth h], IA [auth i], KA [auth k], N, NA [auth n], O, PA [auth p], Q, T, V, X, Y | 2 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G42666HT GlyCosmos: G42666HT GlyGen: G42666HT | |||||
Entity ID: 7 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | JA [auth j], P, Z | 6 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G56014GC GlyCosmos: G56014GC GlyGen: G56014GC | |||||
Entity ID: 8 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | BA [auth b], LA [auth l], R | 3 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G15407YE GlyCosmos: G15407YE GlyGen: G15407YE | |||||
Entity ID: 9 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-3)]alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | CA [auth c], MA [auth m], S | 10 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G40702WU GlyCosmos: G40702WU GlyGen: G40702WU | |||||
Entity ID: 10 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | EA [auth e], OA [auth o], U | 6 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G01760ZU GlyCosmos: G01760ZU GlyGen: G01760ZU | |||||
Small Molecules
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | AB [auth C] BB [auth G] CB [auth D] DB [auth D] EB [auth D] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
Experimental Data & Validation
Experimental Data
- Method: ELECTRON MICROSCOPY
- Resolution: 4.42 Å
- Aggregation State: PARTICLE
- Reconstruction Method: SINGLE PARTICLE
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.12 |
| MODEL REFINEMENT | Coot | 0.8.9 |
| RECONSTRUCTION | RELION | 2.1 |
Entry History & Funding Information
Deposition Data
- Released Date: 2019-11-06 Deposition Author(s): Ozorowski, G., Steichen, J.M., Schief, W.R., Ward, A.B.
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | UM1 AI100663 |
| Bill & Melinda Gates Foundation | United States | OPP1115782 |
| Bill & Melinda Gates Foundation | United States | OPP1084519 |
Revision History (Full details and data files)
- Version 1.0: 2019-11-06
Type: Initial release - Version 1.1: 2019-11-13
Changes: Data collection, Database references - Version 1.2: 2019-12-18
Changes: Author supporting evidence, Database references, Other - Version 2.0: 2020-07-29
Type: Remediation
Reason: Carbohydrate remediation
Changes: Atomic model, Data collection, Derived calculations, Structure summary













