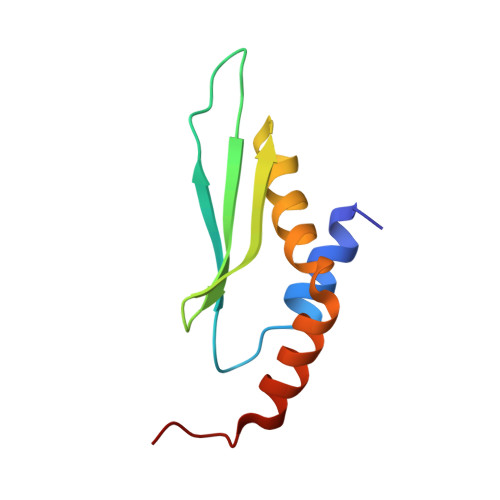
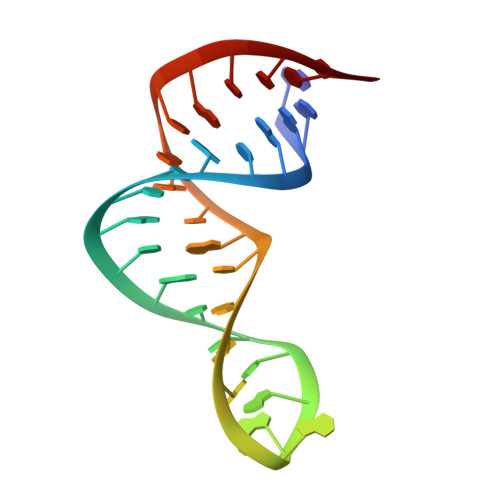
Structure of a Yeast RNase III dsRBD Complex with a Noncanonical RNA Substrate Provides New Insights into Binding Specificity of dsRBDs.
Wang, Z., Hartman, E., Roy, K., Chanfreau, G., Feigon, J.(2011) Structure 19: 999-1010
- PubMed: 21742266
- DOI: https://doi.org/10.1016/j.str.2011.03.022
- Primary Citation of Related Structures:
2LBS, 2LUP - PubMed Abstract:
dsRBDs often bind dsRNAs with some specificity, yet the basis for this is poorly understood. Rnt1p, the major RNase III in Saccharomyces cerevisiae, cleaves RNA substrates containing hairpins capped by A/uGNN tetraloops, using its dsRBD to recognize a conserved tetraloop fold. However, the identification of a Rnt1p substrate with an AAGU tetraloop raised the question of whether Rnt1p binds to this noncanonical substrate differently than to A/uGNN tetraloops. The solution structure of Rnt1p dsRBD bound to an AAGU-capped hairpin reveals that the tetraloop undergoes a structural rearrangement upon binding to Rnt1p dsRBD to adopt a backbone conformation that is essentially the same as the AGAA tetraloop, and indicates that a conserved recognition mode is used for all Rnt1p substrates. Comparison of free and RNA-bound Rnt1p dsRBD reveals that tetraloop-specific binding requires a conformational change in helix α1. Our findings provide a unified model of binding site selection by this dsRBD.
Organizational Affiliation:
Department of Chemistry and Biochemistry, P.O. Box 951569, University of California, Los Angeles, CA 90095-1569, USA.