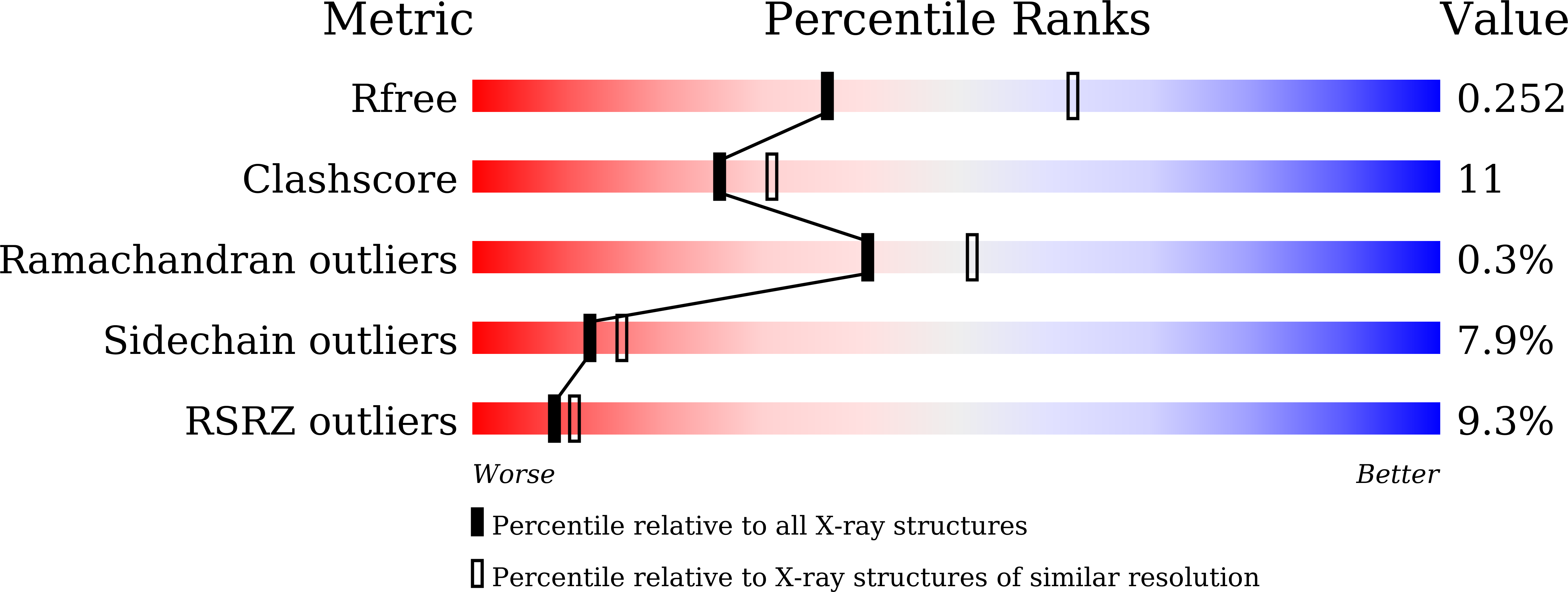
Molecular Characteristics of Repotrectinib That Enable Potent Inhibition of TRK Fusion Proteins and Resistant Mutations.
Murray, B.W., Rogers, E., Zhai, D., Deng, W., Chen, X., Sprengeler, P.A., Zhang, X., Graber, A., Reich, S.H., Stopatschinskaja, S., Solomon, B., Besse, B., Drilon, A.(2021) Mol Cancer Ther 20: 2446-2456
- PubMed: 34625502
- DOI: https://doi.org/10.1158/1535-7163.MCT-21-0632
- Primary Citation of Related Structures:
7VKM, 7VKN, 7VKO - PubMed Abstract:
NTRK chromosomal rearrangements yield oncogenic TRK fusion proteins that are sensitive to TRK inhibitors (larotrectinib and entrectinib) but often mutate, limiting the durability of response for NTRK + patients. Next-generation inhibitors with compact macrocyclic structures (repotrectinib and selitrectinib) were designed to avoid resistance mutations. Head-to-head potency comparisons of TRK inhibitors and molecular characterization of binding interactions are incomplete, obscuring a detailed understanding of how molecular characteristics translate to potency. Larotrectinib, entrectinib, selitrectinib, and repotrectinib were characterized using cellular models of wild-type TRKA/B/C fusions and resistance mutant variants with a subset evaluated in xenograft tumor models. Crystal structures were determined for repotrectinib bound to TRKA (wild-type, solvent-front mutant). TKI-naïve and pretreated case studies are presented. Repotrectinib was the most potent inhibitor of wild-type TRKA/B/C fusions and was more potent than selitrectinib against all tested resistance mutations, underscoring the importance of distinct features of the macrocycle structures. Cocrystal structures of repotrectinib with wild-type TRKA and the TRKA G595R SFM variant elucidated how differences in macrocyclic inhibitor structure, binding orientation, and conformational flexibility affect potency and mutant selectivity. The SFM crystal structure revealed an unexpected intramolecular arginine sidechain interaction. Repotrectinib caused tumor regression in LMNA-NTRK1 xenograft models harboring GKM, SFM, xDFG, and GKM + SFM compound mutations. Durable responses were observed in TKI-naïve and -pretreated patients with NTRK + cancers treated with repotrectinib (NCT03093116). This comprehensive analysis of first- and second-generation TRK inhibitors informs the clinical utility, structural determinants of inhibitor potency, and design of new generations of macrocyclic inhibitors.
Organizational Affiliation:
Turning Point Therapeutics, San Diego, California. brion.murray@tptherapeutics.com.