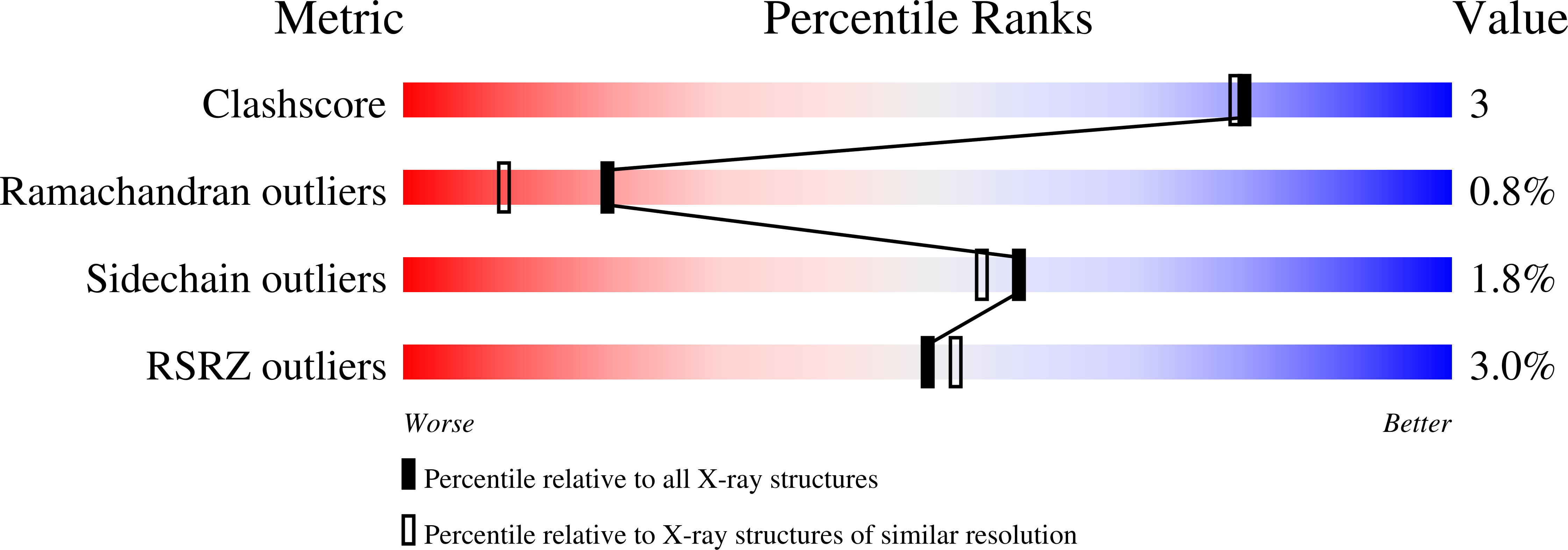
Arginine residues at internal positions in a protein are always charged.
Harms, M.J., Schlessman, J.L., Sue, G.R., Garcia-Moreno E, B.(2011) Proc Natl Acad Sci U S A 108: 18954-18959
- PubMed: 22080604
- DOI: https://doi.org/10.1073/pnas.1104808108
- Primary Citation of Related Structures:
3D4W, 3D8G, 3DHQ - PubMed Abstract:
Many functionally essential ionizable groups are buried in the hydrophobic interior of proteins. A systematic study of Lys, Asp, and Glu residues at 25 internal positions in staphylococcal nuclease showed that their pK(a) values can be highly anomalous, some shifted by as many as 5.7 pH units relative to normal pK(a) values in water. Here we show that, in contrast, Arg residues at the same internal positions exhibit no detectable shifts in pK(a); they are all charged at pH ≤ 10. Twenty-three of these 25 variants with Arg are folded at both pH 7 and 10. The mean decrease in thermodynamic stability from substitution with Arg was 6.2 kcal/mol at this pH, comparable to that for substitution with Lys, Asp, or Glu at pH 7. The physical basis behind the remarkable ability of Arg residues to remain protonated in environments otherwise incompatible with charges is suggested by crystal structures of three variants showing how the guanidinium moiety of the Arg side chain is effectively neutralized through multiple hydrogen bonds to protein polar atoms and to site-bound water molecules. The length of the Arg side chain, and slight deformations of the protein, facilitate placement of the guanidinium moieties near polar groups or bulk water. This unique capacity of Arg side chains to retain their charge in dehydrated environments likely contributes toward the important functional roles of internal Arg residues in situations where a charge is needed in the interior of a protein, in a lipid bilayer, or in similarly hydrophobic environments.
Organizational Affiliation:
Department of Biophysics, Johns Hopkins University, 3400 North Charles Street, Baltimore, MD 21218, USA.