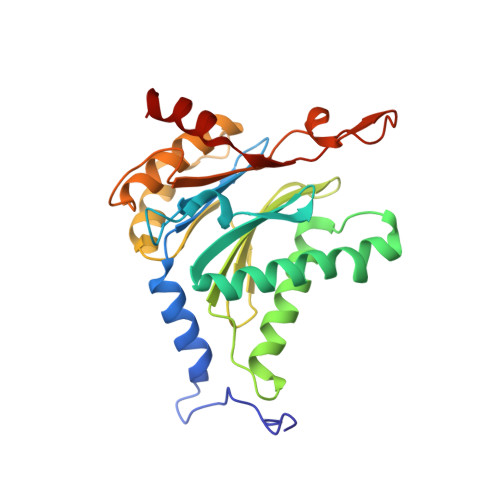
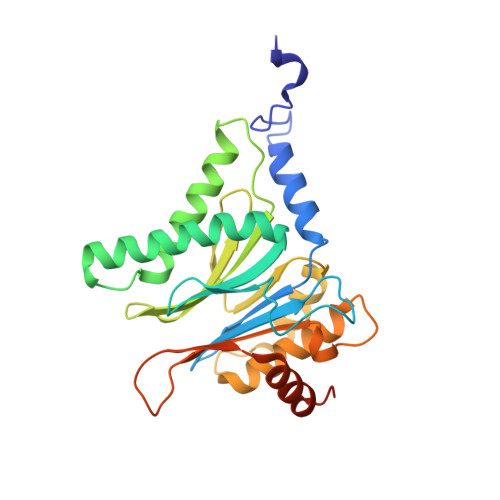
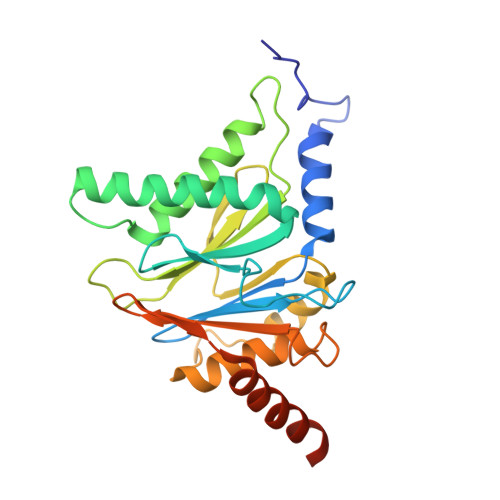
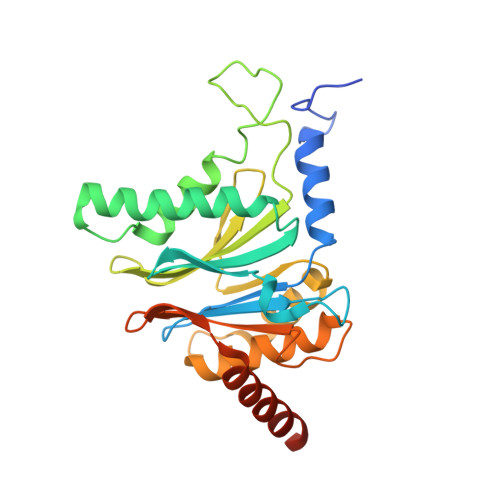
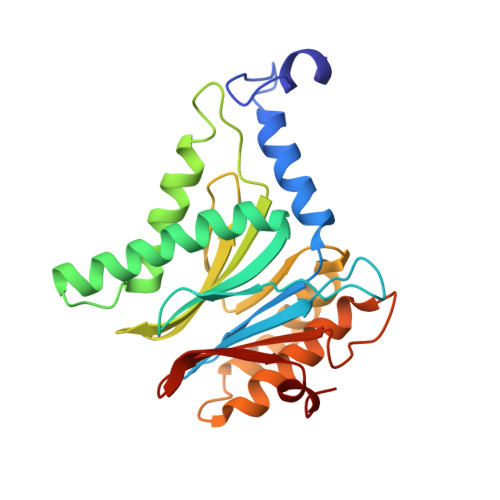
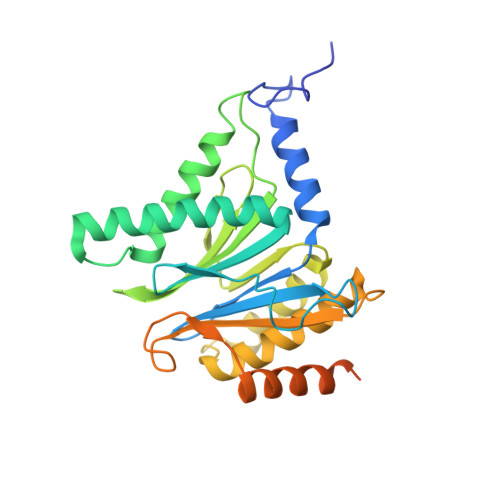
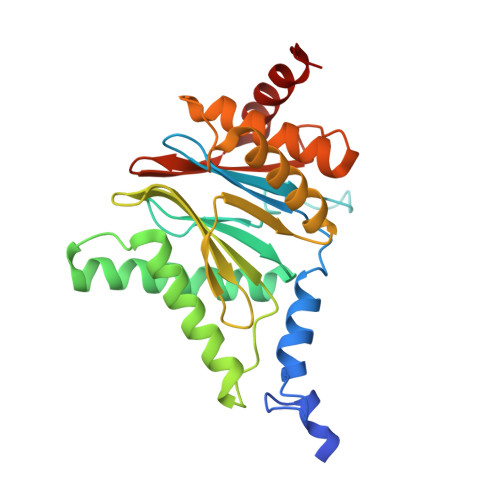
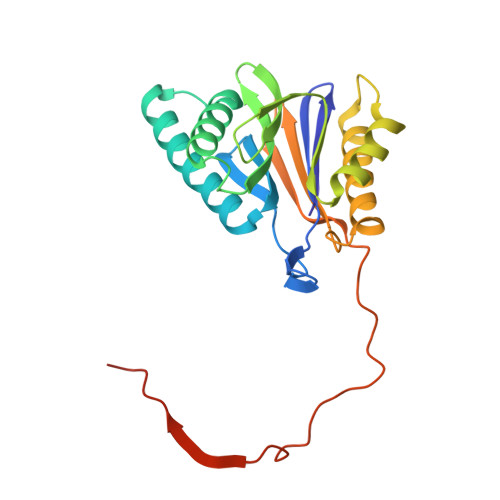
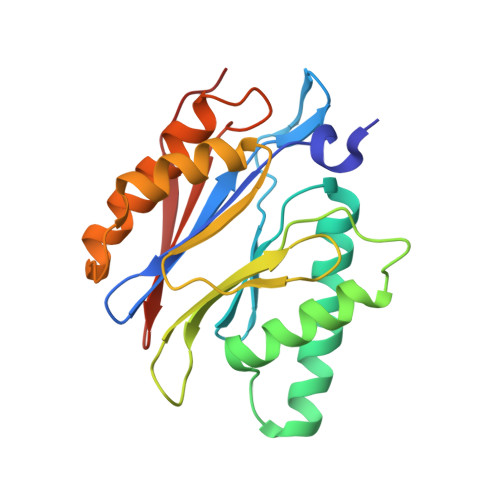
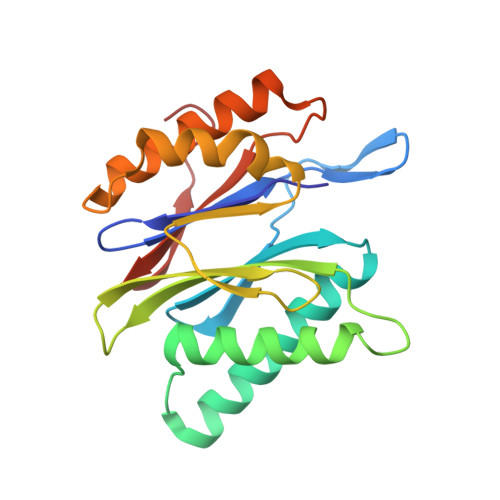
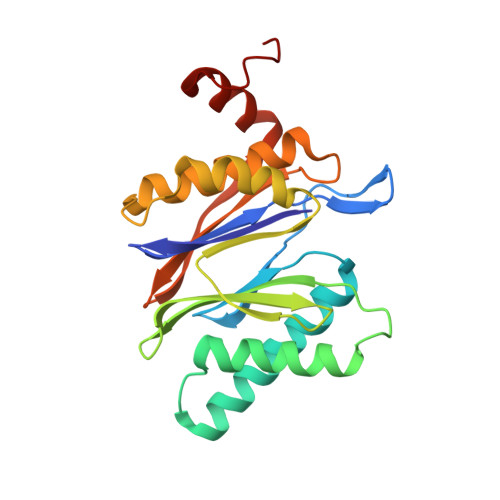
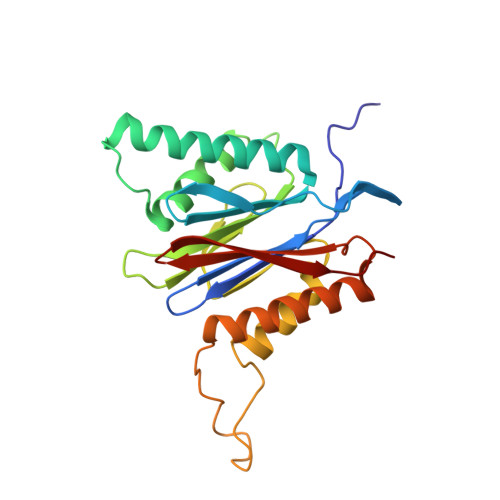
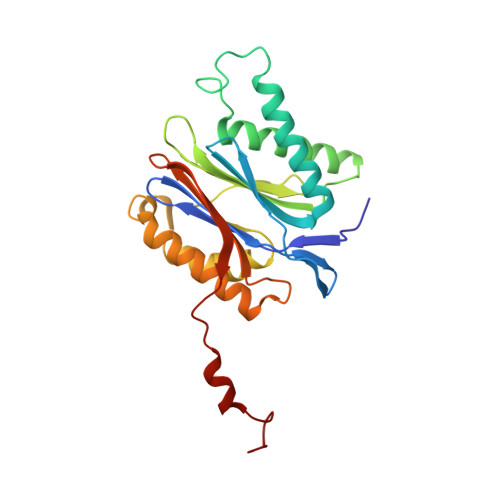
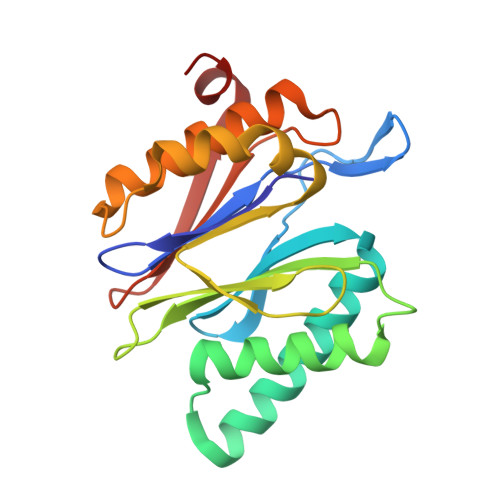
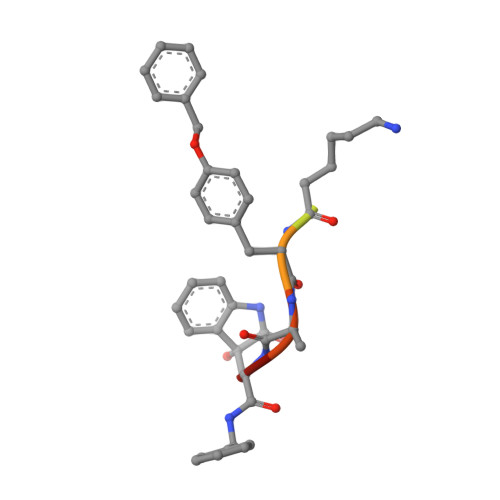
Dimerized linear mimics of a natural cyclopeptide (TMC-95A) are potent noncovalent inhibitors of the eukaryotic 20S proteasome
Desvergne, A., Genin, E., Marechal, X., Gallastegui, N., Dufau, L., Richy, N., Groll, M., Vidal, J., Reboud-Ravaux, M.(2013) J Med Chem 56: 3367-3378
- PubMed: 23540790
- DOI: https://doi.org/10.1021/jm4002007
- Primary Citation of Related Structures:
4JSQ, 4JSU, 4JT0 - PubMed Abstract:
Noncovalent proteasome inhibitors introduce an alternative mechanism of inhibition to that of covalent inhibitors used in cancer therapy. Starting from a noncovalent linear mimic of TMC-95A, a series of dimerized inhibitors using polyaminohexanoic acid spacers has been designed and optimized to target simultaneously two of the six active sites of the eukaryotic 20S proteasome. The homodimerized compounds actively inhibited chymotrypsin-like (Ki = 6-11 nM) and trypsin-like activities, whereas postacid activity was poorly modified. The noncovalent binding mode was ascertained by X-ray crystallography of the inhibitors complexed with the yeast 20S proteasome. The inhibition of proteasomal activities in human cells was evaluated. The use of the multivalency inhibitor concept has produced highly efficient and selective noncovalent compounds (no inhibition of calpain and cathepsin) that have potential therapeutic advantages compared to covalent binders such as bortezomib and carfilzomib.
Organizational Affiliation:
Enzymologie Moléculaire et Fonctionnelle, UR4, University Paris 6, Pierre et Marie Curie, UPMC-Sorbonne Universités, Case 256, 7 Quai Saint Bernard, 75252 Paris Cedex 05, France.