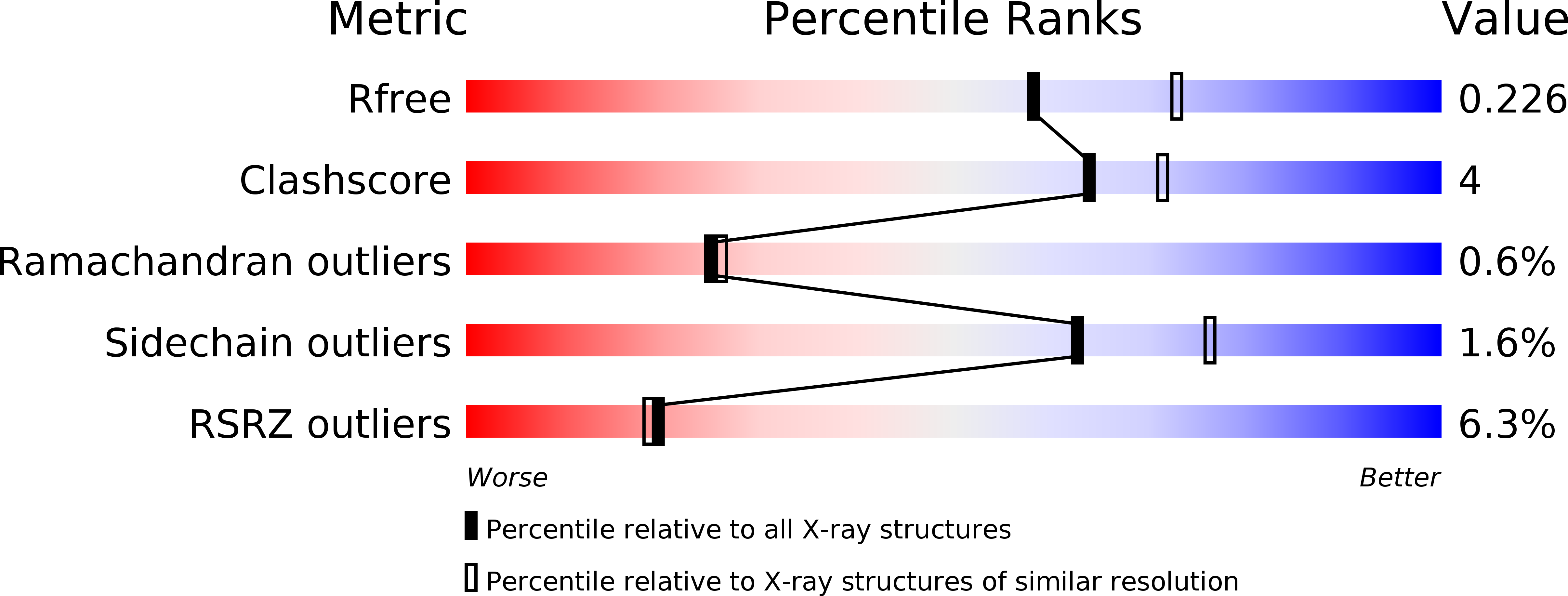
The plant defense signal galactinol is specifically used as a nutrient by the bacterial pathogenAgrobacterium fabrum.
Meyer, T., Vigouroux, A., Aumont-Nicaise, M., Comte, G., Vial, L., Lavire, C., Morera, S.(2018) J Biol Chem 293: 7930-7941
- PubMed: 29602905
- DOI: https://doi.org/10.1074/jbc.RA118.001856
- Primary Citation of Related Structures:
6EPY, 6EPZ, 6EQ0, 6EQ1, 6EQ8 - PubMed Abstract:
The bacterial plant pathogen Agrobacterium fabrum uses periplasmic-binding proteins (PBPs) along with ABC transporters to import a wide variety of plant molecules as nutrients. Nonetheless, how A. fabrum acquires plant metabolites is incompletely understood. Using genetic approaches and affinity measurements, we identified here the PBP MelB and its transporter as being responsible for the uptake of the raffinose family of oligosaccharides (RFO), which are the most widespread d-galactose-containing oligosaccharides in higher plants. We also found that the RFO precursor galactinol, recently described as a plant defense molecule, is imported into Agrobacterium via MelB with nanomolar range affinity. Structural analyses and binding mode comparisons of the X-ray structures of MelB in complex with raffinose, stachyose, galactinol, galactose, and melibiose (a raffinose degradation product) revealed how MelB recognizes the nonreducing end galactose common to all these ligands and that MelB has a strong preference for a two-unit sugar ligand. Of note, MelB conferred a competitive advantage to A. fabrum in colonizing the rhizosphere of tomato plants. Our integrative work highlights the structural and functional characteristics of melibiose and galactinol assimilation by A. fabrum , leading to a competitive advantage for these bacteria in the rhizosphere. We propose that the PBP MelB, which is highly conserved among both symbionts and pathogens from Rhizobiace family, is a major trait in these bacteria required for early steps of plant colonization.
Organizational Affiliation:
UMR Ecologie Microbienne, CNRS, INRA, VetAgro Sup, UCBL, Université de Lyon, F-69622, Villeurbanne, Lyon, France.