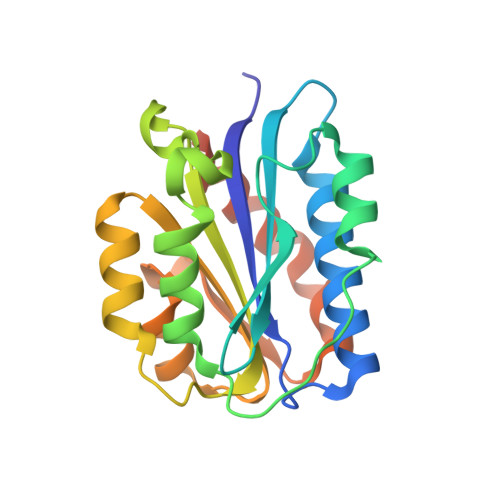
Structure of a collagen VI alpha 3 chain VWA domain array: adaptability and functional implications of myopathy causing mutations.
Solomon-Degefa, H., Gebauer, J.M., Jeffries, C.M., Freiburg, C.D., Meckelburg, P., Bird, L.E., Baumann, U., Svergun, D.I., Owens, R.J., Werner, J.M., Behrmann, E., Paulsson, M., Wagener, R.(2020) J Biol Chem 295: 12755-12771
- PubMed: 32719005
- DOI: https://doi.org/10.1074/jbc.RA120.014865
- Primary Citation of Related Structures:
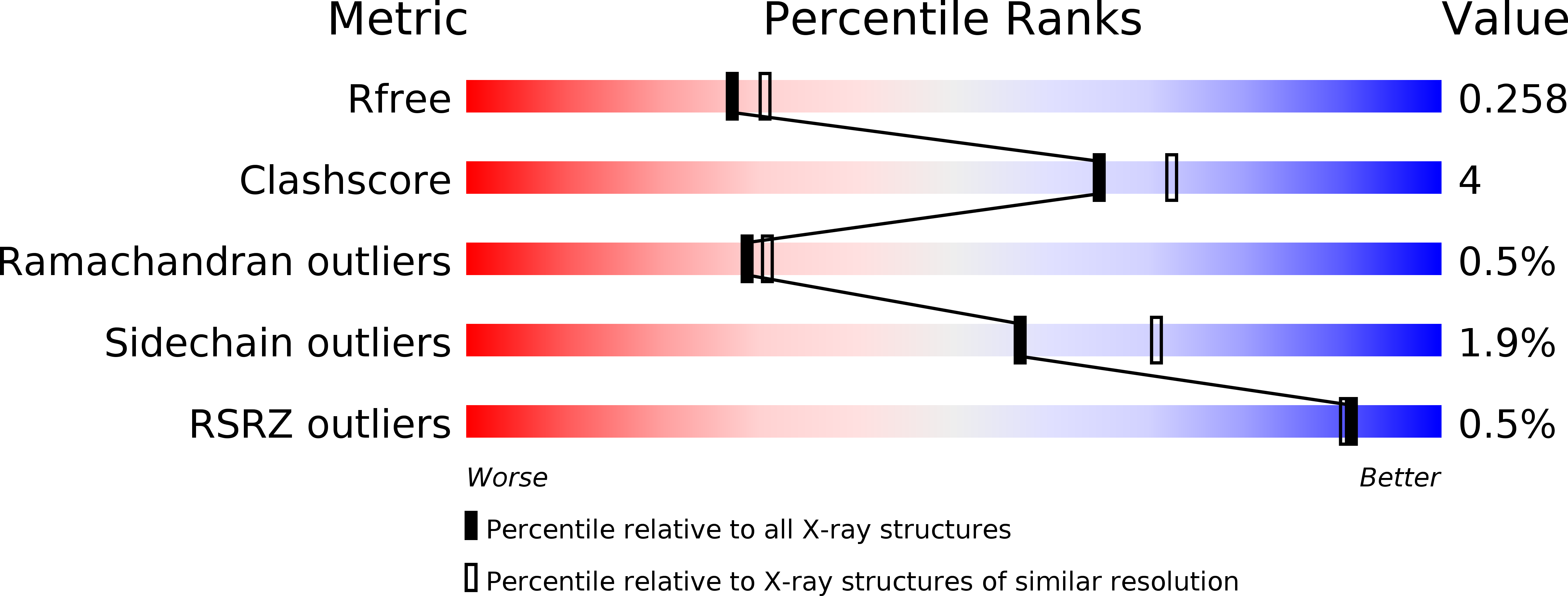
6SNK - PubMed Abstract:
Collagen VI is a ubiquitous heterotrimeric protein of the extracellular matrix (ECM) that plays an essential role in the proper maintenance of skeletal muscle. Mutations in collagen VI lead to a spectrum of congenital myopathies, from the mild Bethlem myopathy to the severe Ullrich congenital muscular dystrophy. Collagen VI contains only a short triple helix and consists primarily of von Willebrand factor type A (VWA) domains, protein-protein interaction modules found in a range of ECM proteins. Disease-causing mutations occur commonly in the VWA domains, and the second VWA domain of the α3 chain, the N2 domain, harbors several such mutations. Here, we investigate structure-function relationships of the N2 mutations to shed light on their possible myopathy mechanisms. We determined the X-ray crystal structure of N2, combined with monitoring secretion efficiency in cell culture of selected N2 single-domain mutants, finding that mutations located within the central core of the domain severely affect secretion efficiency. In longer α3 chain constructs, spanning N6-N3, small-angle X-ray scattering demonstrates that the tandem VWA array has a modular architecture and samples multiple conformations in solution. Single-particle EM confirmed the presence of multiple conformations. Structural adaptability appears intrinsic to the VWA domain region of collagen VI α3 and has implications for binding interactions and modulating stiffness within the ECM.
Organizational Affiliation:
Center for Biochemistry, Medical Faculty, University of Cologne, Cologne, Germany.