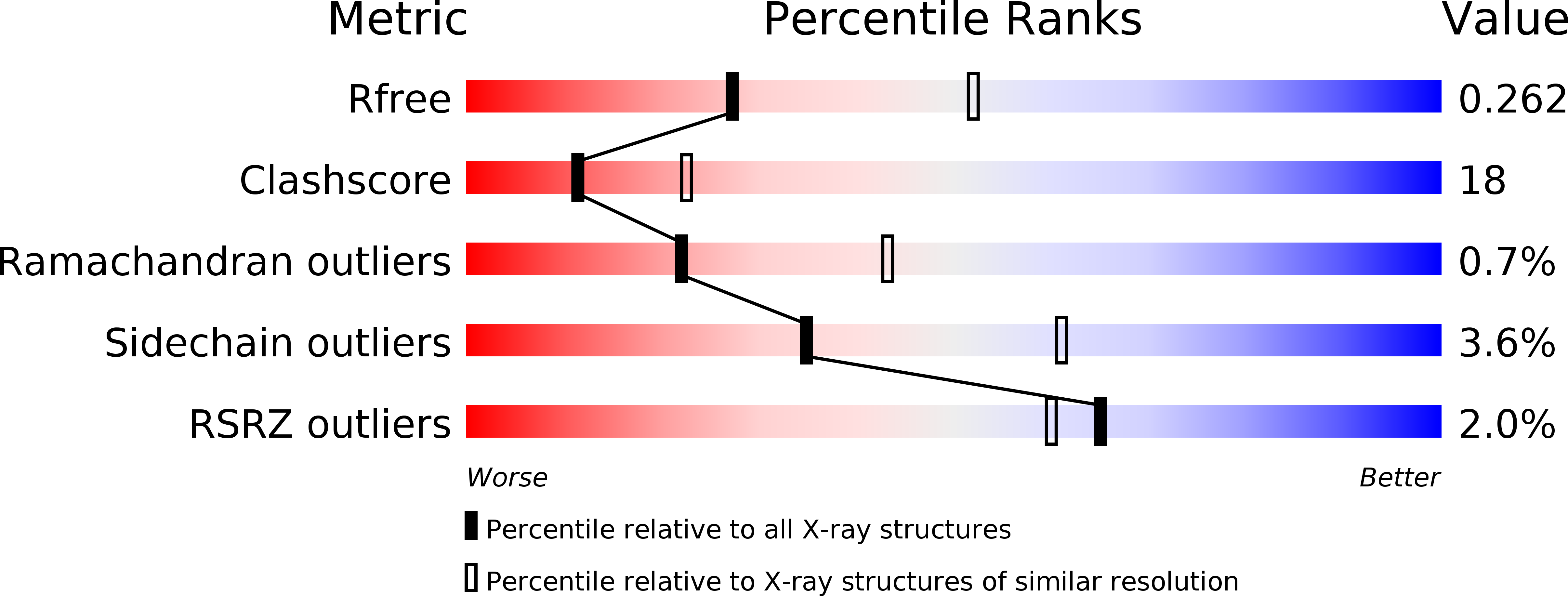
A snapshot of a transition state analogue of a novel thermophilic esterase belonging to the subfamily of mammalian hormone-sensitive lipase.
De Simone, G., Galdiero, S., Manco, G., Lang, D., Rossi, M., Pedone, C.(2000) J Mol Biology 303: 761-771
- PubMed: 11061974
- DOI: https://doi.org/10.1006/jmbi.2000.4195
- Primary Citation of Related Structures:
1EVQ - PubMed Abstract:
EST2 is a novel thermophilic carboxylesterase, isolated and cloned from Alicyclobacillus (formerly Bacillus) acidocaldarius, which optimally hydrolyses esters with acyl chain lengths of six to eight carbon atoms at 70 degrees C. On the basis of the amino acid sequence homology, it has been classified as a member of the mammalian hormone-sensitive lipase (HSL) subfamily. The crystal structure of EST2, complexed with a sulphonyl derivative, has been determined at 2.6 A resolution by a multiple wavelength anomalous diffraction experiment on a seleno-methionine derivative. EST2 presents a canonical alpha/beta hydrolase core, shielded at the C-terminal side by a cap region built up of five helices. It contains the lipase-like catalytic triad, Ser155, His282 and Asp252, whereby the nucleophile is covalently modified. This allows an unambiguous view of the putative active site of EST2, detecting the oxyanion hole, in whose formation the amino acid sequence motif His81-Gly82-Gly83-Gly84 is involved, and the hydrophobic binding pocket for the acyl chain. The structural model here reported provides the first example of a transition state analogue of an esterase/lipase belonging to the HSL group, thus affording useful information for the design of medical inhibitors. Moreover, as the first X-ray structure of a thermophilic carboxylesterase, the comparison with its mesophilic homologue, the Brefeldin A esterase (BFAE) from Bacillus subtilis, allows the identification of putative determinants of thermal stability.
Organizational Affiliation:
Dipartimento di Chimica and Centro di Studio di Biocristallografia- CNR, University of Naples "Federico II", via Mezzocannone 8, Naples, 80134, Italy.