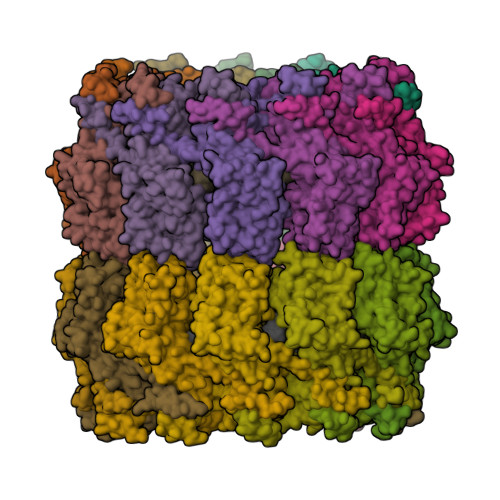
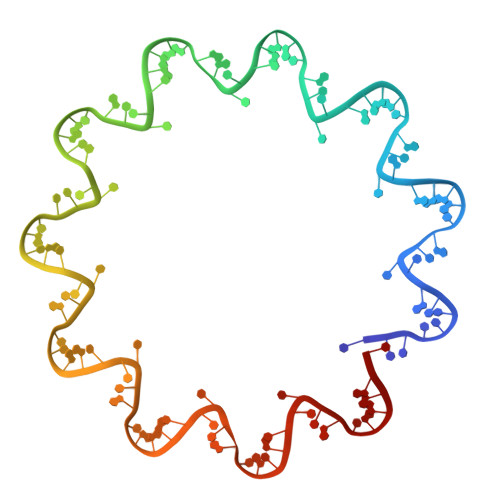
Crystal Structure of the Rabies Virus Nucleoprotein-RNA Complex
Albertini, A.A.V., Wernimont, A.K., Muziol, T., Ravelli, R.B.G., Clapier, C.R., Schoehn, G., Weissenhorn, W., Ruigrok, R.W.H.(2006) Science 313: 360-363
- PubMed: 16778023
- DOI: https://doi.org/10.1126/science.1125280
- Primary Citation of Related Structures:
2GTT - PubMed Abstract:
Negative-strand RNA viruses condense their genome into a helical nucleoprotein-RNA complex, the nucleocapsid, which is packed into virions and serves as a template for the RNA-dependent RNA polymerase complex. The crystal structure of a recombinant rabies virus nucleoprotein-RNA complex, organized in an undecameric ring, has been determined at 3.5 angstrom resolution. Polymerization of the nucleoprotein is achieved by domain exchange between protomers, with flexible hinges allowing nucleocapsid formation. The two core domains of the nucleoprotein clamp around the RNA at their interface and shield it from the environment. RNA sequestering by nucleoproteins is likely a common mechanism used by negative-strand RNA viruses to protect their genomes from the innate immune response directed against viral RNA in human host cells at certain stages of an infectious cycle.
Organizational Affiliation:
Institut de Virologie Moléculaire et Structurale, FRE 2854 Université Joseph Fourier-CNRS, Boite Postale 181, 38042 Grenoble, France.