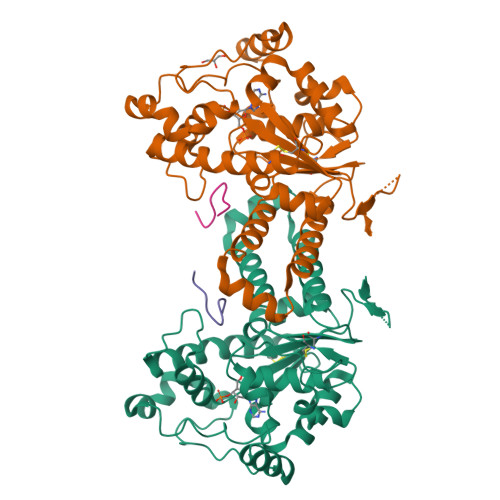
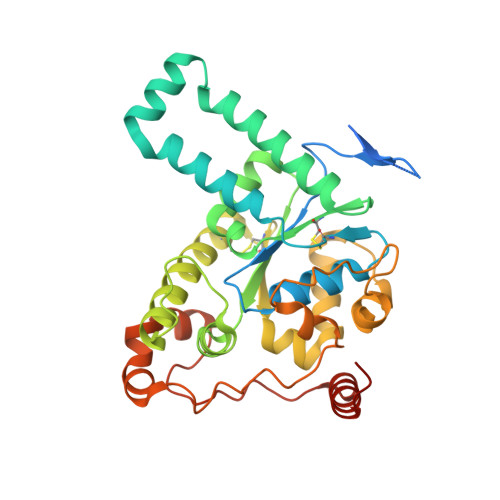
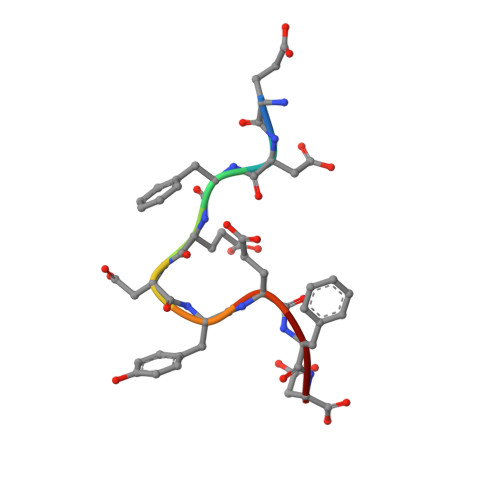
Crystal structure of human tyrosylprotein sulfotransferase-2 reveals the mechanism of protein tyrosine sulfation reaction.
Teramoto, T., Fujikawa, Y., Kawaguchi, Y., Kurogi, K., Soejima, M., Adachi, R., Nakanishi, Y., Mishiro-Sato, E., Liu, M.C., Sakakibara, Y., Suiko, M., Kimura, M., Kakuta, Y.(2013) Nat Commun 4: 1572-1572
- PubMed: 23481380
- DOI: https://doi.org/10.1038/ncomms2593
- Primary Citation of Related Structures:
3AP1, 3AP3 - PubMed Abstract:
Post-translational protein modification by tyrosine sulfation has an important role in extracellular protein-protein interactions. The protein tyrosine sulfation reaction is catalysed by the Golgi enzyme called the tyrosylprotein sulfotransferase. To date, no crystal structure is available for tyrosylprotein sulfotransferase. Detailed mechanism of protein tyrosine sulfation reaction has thus remained unclear. Here we present the first crystal structure of the human tyrosylprotein sulfotransferase isoform 2 complexed with a substrate peptide (C4P5Y3) derived from complement C4 and 3'-phosphoadenosine-5'-phosphate at 1.9 Å resolution. Structural and complementary mutational analyses revealed the molecular basis for catalysis being an SN2-like in-line displacement mechanism. Tyrosylprotein sulfotransferase isoform 2 appeared to recognize the C4 peptide in a deep cleft by using a short parallel β-sheet type interaction, and the bound C4P5Y3 forms an L-shaped structure. Surprisingly, the mode of substrate peptide recognition observed in the tyrosylprotein sulfotransferase isoform 2 structure resembles that observed for the receptor type tyrosine kinases.
Organizational Affiliation:
Laboratory of Structural Biology, Graduate School of Systems Life Sciences, Kyushu University, Hakozaki 6-10-1, Fukuoka 812-8581, Japan.