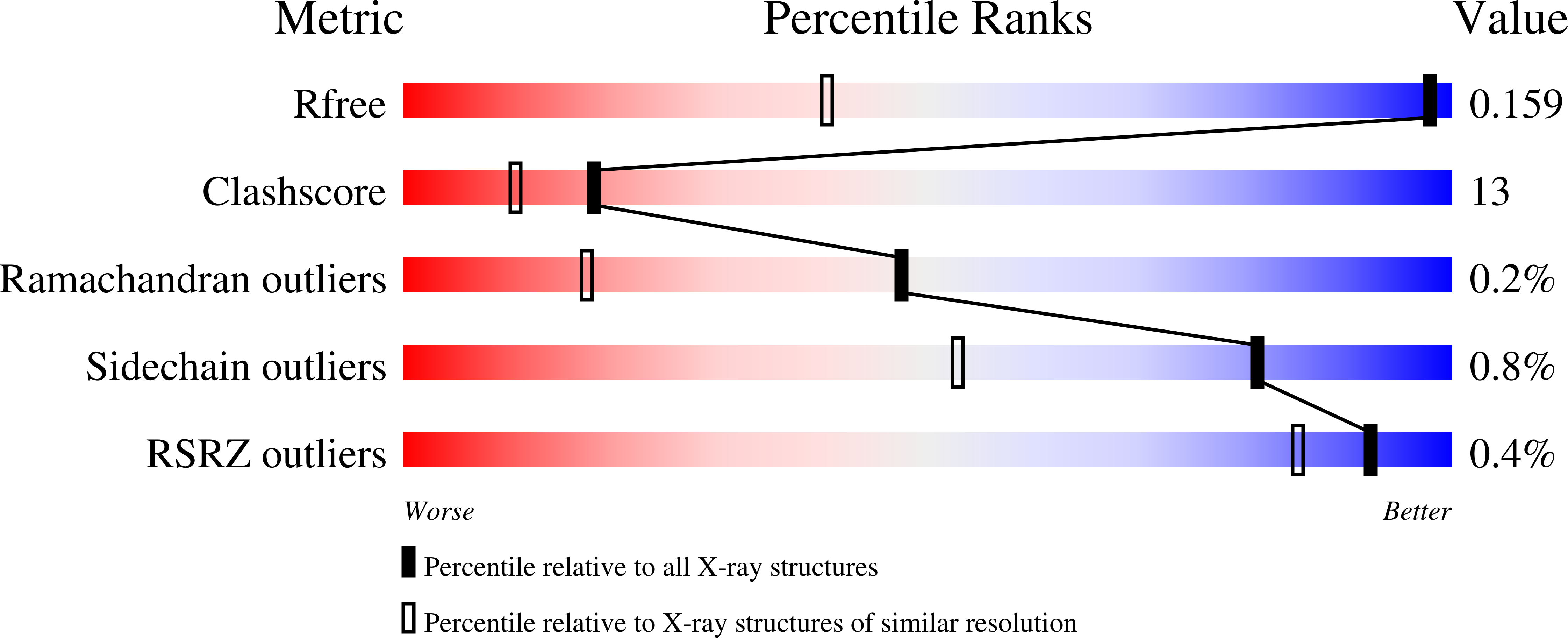
Distortion of flavin geometry is linked to ligand binding in cholesterol oxidase
Lyubimov, A.Y., Heard, K., Tang, H., Sampson, N.S., Vrielink, A.(2007) Protein Sci 16: 2647-2656
- PubMed: 18029419
- DOI: https://doi.org/10.1110/ps.073168207
- Primary Citation of Related Structures:
3B3R, 3B6D - PubMed Abstract:
Two high-resolution structures of a double mutant of bacterial cholesterol oxidase in the presence or absence of a ligand, glycerol, are presented, showing the trajectory of glycerol as it binds in a Michaelis complex-like position in the active site. A group of three aromatic residues forces the oxidized isoalloxazine moiety to bend along the N5-N10 axis as a response to the binding of glycerol in the active site. Movement of these aromatic residues is only observed in the glycerol-bound structure, indicating that some tuning of the FAD redox potential is caused by the formation of the Michaelis complex during regular catalysis. This structural study suggests a possible mechanism of substrate-assisted flavin activation, improves our understanding of the interplay between the enzyme, its flavin cofactor and its substrate, and is of use to the future design of effective cholesterol oxidase inhibitors.
Organizational Affiliation:
Department of Molecular Cell and Developmental Biology, University of California, Santa Cruz, California 95064, USA.