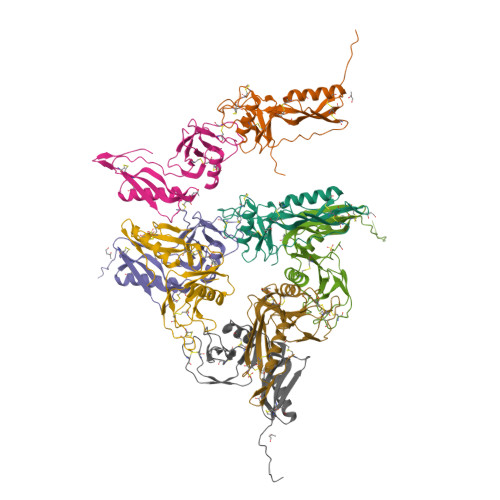
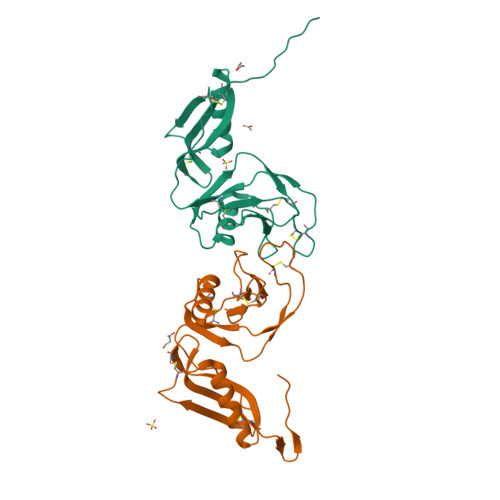
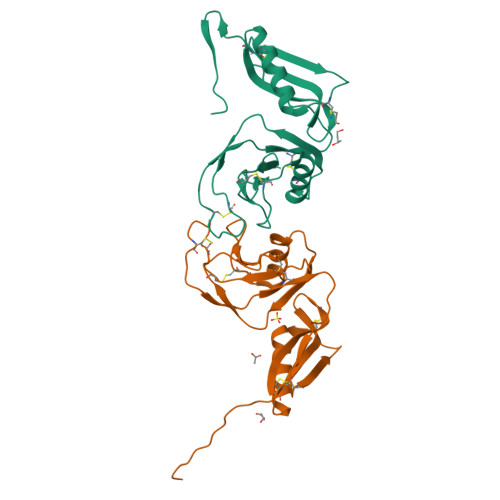
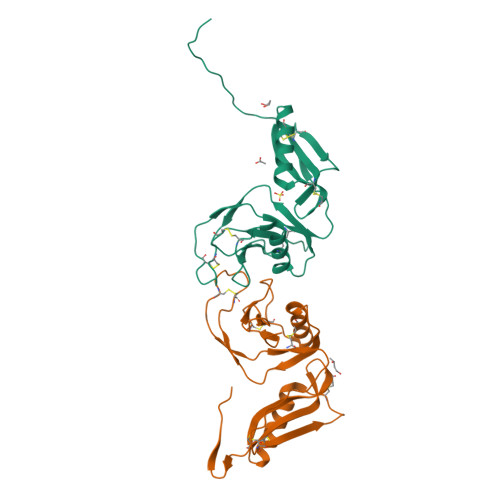
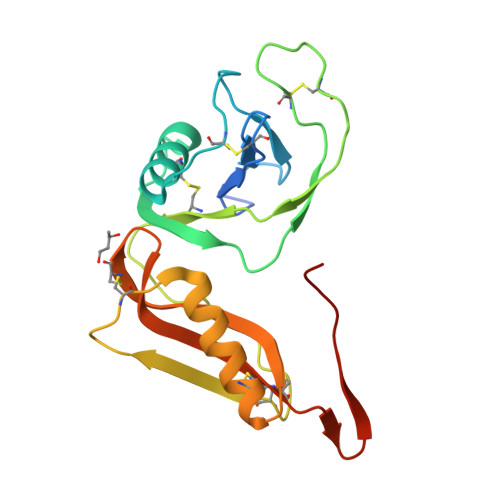
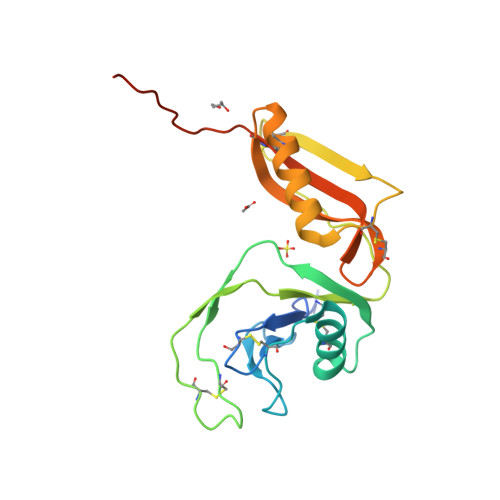
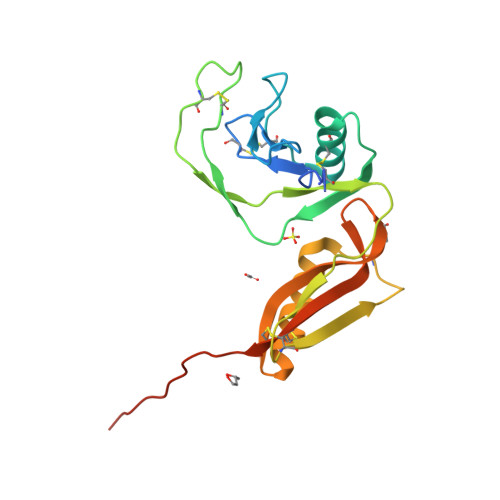
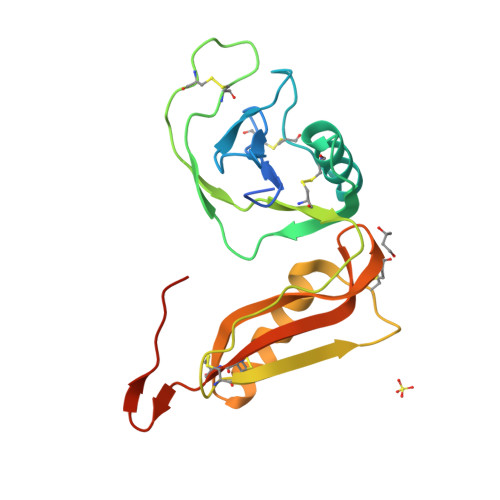
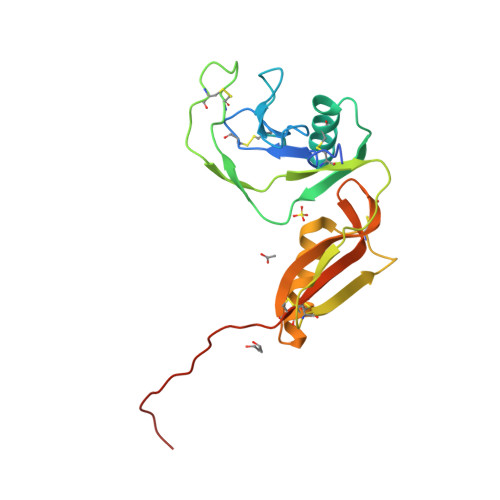
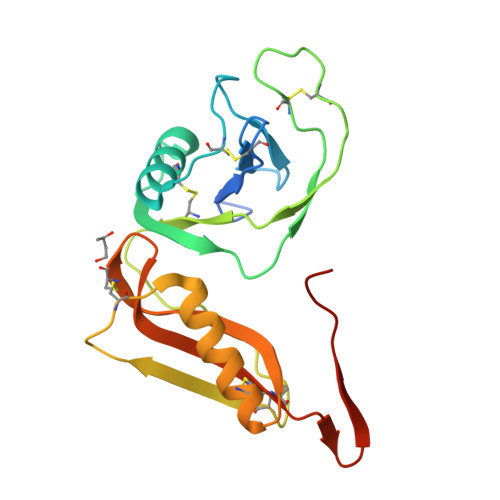
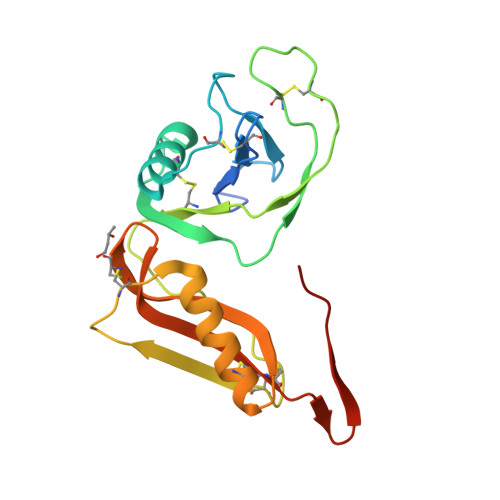
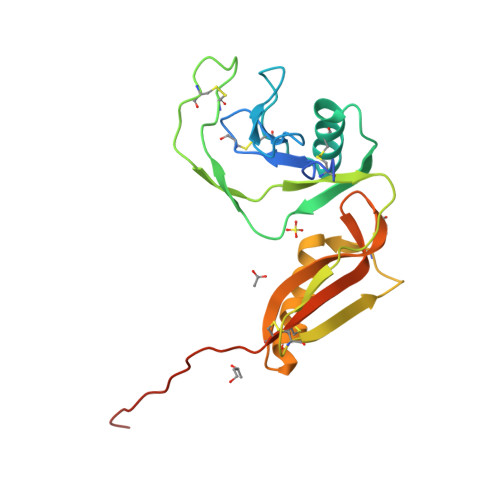
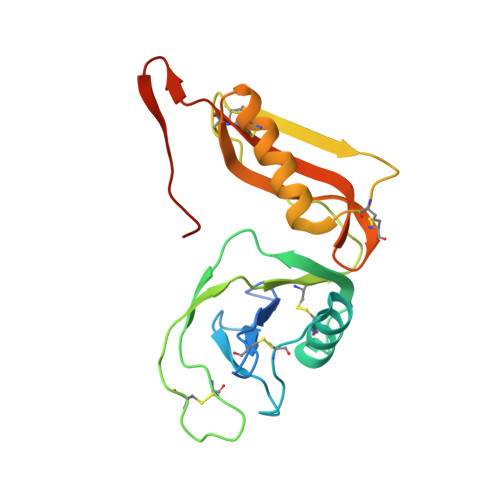
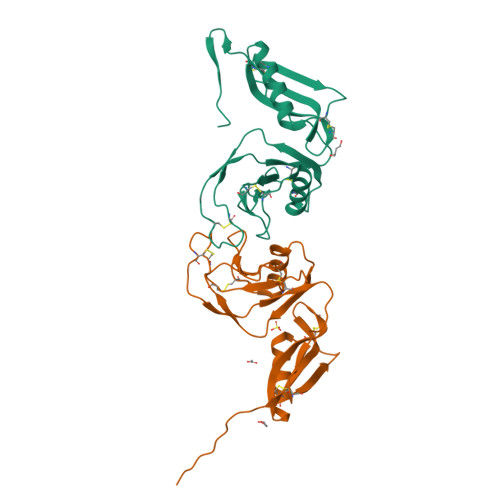Structure and biochemical analysis of the heparin-induced E1 dimer of the amyloid precursor protein.
Dahms, S.O., Hoefgen, S., Roeser, D., Schlott, B., Guhrs, K.H., Than, M.E.(2010) Proc Natl Acad Sci U S A 107: 5381-5386
- PubMed: 20212142
- DOI: https://doi.org/10.1073/pnas.0911326107
- Primary Citation of Related Structures:
3KTM - PubMed Abstract:
The amyloid precursor protein (APP) is the key player in Alzheimer's disease pathology, yet APP and its analogues are also essential for neuronal development and cell homeostasis in mammals. We have determined the crystal structure of the entire N-terminal APP-E1 domain consisting of the growth factor like and the copper binding domains at 2.7-A resolution and show that E1 functions as a rigid functional entity. The two subdomains interact tightly in a pH-dependent manner via an evolutionarily conserved interface area. Two E1 entities dimerize upon their interaction with heparin, requiring 8-12 sugar rings to form the heparin-bridged APP-E1 dimer in an endothermic and pH-dependent process that is characterized by a low micromolar dissociation constant. Limited proteolysis confirms that the heparin-bridged E1 dimers obtained in solution correspond to a dimer contact in our crystal, enabling us to model this heparin-[APP-E1](2) complex. Correspondingly, the APP-based signal transduction, cell-cell- and/or cell-ECM interaction should depend on dimerization induced by heparin, as well as on pH, arguing that APP could fulfill different functions depending on its (sub)cellular localization.
Organizational Affiliation:
Leibniz Institute for Age Research-Fritz Lipman Institute, Protein Crystallography Group, Jena, Germany.