Membrane remodeling by the double-barrel scaffolding protein of poxvirus.
Hyun, J.K., Accurso, C., Hijnen, M., Schult, P., Pettikiriarachchi, A., Mitra, A.K., Coulibaly, F.(2011) PLoS Pathog 7: e1002239-e1002239
- PubMed: 21931553
- DOI: https://doi.org/10.1371/journal.ppat.1002239
- Primary Citation of Related Structures:
3SAM, 3SAQ - PubMed Abstract:
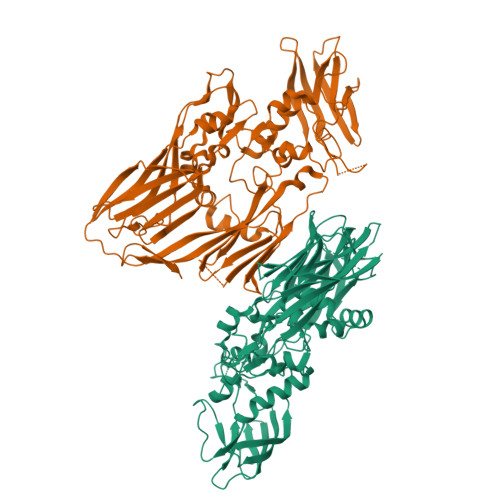
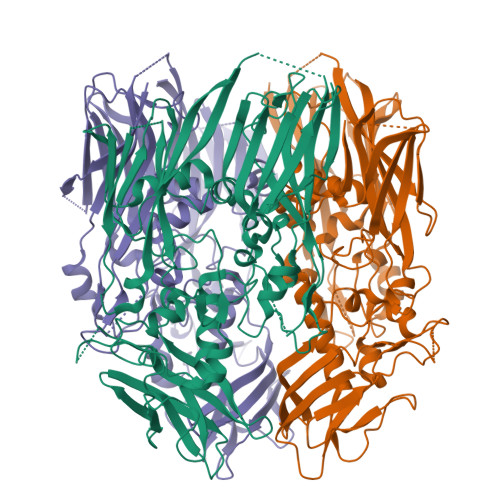
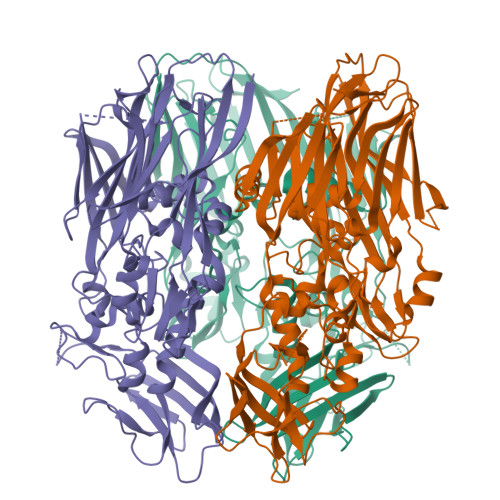
In contrast to most enveloped viruses, poxviruses produce infectious particles that do not acquire their internal lipid membrane by budding through cellular compartments. Instead, poxvirus immature particles are generated from atypical crescent-shaped precursors whose architecture and composition remain contentious. Here we describe the 2.6 Å crystal structure of vaccinia virus D13, a key structural component of the outer scaffold of viral crescents. D13 folds into two jellyrolls decorated by a head domain of novel fold. It assembles into trimers that are homologous to the double-barrel capsid proteins of adenovirus and lipid-containing icosahedral viruses. We show that, when tethered onto artificial membranes, D13 forms a honeycomb lattice and assembly products structurally similar to the viral crescents and immature particles. The architecture of the D13 honeycomb lattice and the lipid-remodeling abilities of D13 support a model of assembly that exhibits similarities with the giant mimivirus. Overall, these findings establish that the first committed step of poxvirus morphogenesis utilizes an ancestral lipid-remodeling strategy common to icosahedral DNA viruses infecting all kingdoms of life. Furthermore, D13 is the target of rifampicin and its structure will aid the development of poxvirus assembly inhibitors.
Organizational Affiliation:
School of Biological Sciences, the University of Auckland, Auckland, New Zealand.