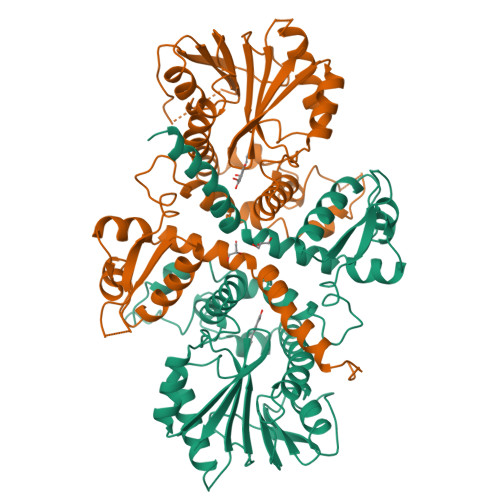
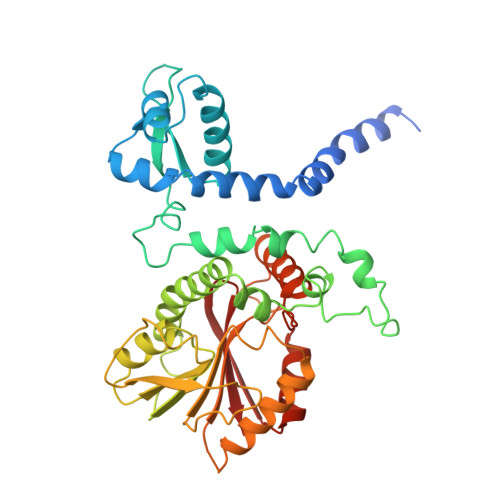
Structural analysis of coniferyl alcohol 9-O-methyltransferase from Linum nodiflorum reveals a novel active-site environment.
Wolters, S., Neeb, M., Berim, A., Schulze Wischeler, J., Petersen, M., Heine, A.(2013) Acta Crystallogr D Biol Crystallogr 69: 888-900
- PubMed: 23633600
- DOI: https://doi.org/10.1107/S0907444913002874
- Primary Citation of Related Structures:
4E70, 4EMS, 4EVI - PubMed Abstract:
Coniferyl alcohol 9-O-methyltransferase from Linum nodiflorum (Linaceae) catalyzes the unusual methylation of the side-chain hydroxyl group of coniferyl alcohol. The protein was heterologously expressed in Escherichia coli as a hexahistidine derivative and purified for crystallization. Diffracting crystals were obtained of the pure protein and of its selenomethionine derivative, as well as of complexes with coniferyl alcohol and with S-adenosyl-L-homocysteine together with coniferyl alcohol 9-O-methyl ether (PDB entries 4ems, 4e70 and 4evi, respectively). The X-ray structures show that the phenylpropanoid binding mode differs from other phenylpropanoid O-methyltransferases such as caffeic acid O-methyltransferase. Moreover, the active site lacks the usually conserved and catalytic histidine residue and thus implies a different reaction mode for methylation. Site-directed mutagenesis was carried out to identify critical amino acids. The binding order of coniferyl alcohol and S-adenosyl-L-methionine was investigated by isothermal titration calorimetry experiments.
Organizational Affiliation:
Institut für Pharmazeutische Biologie und Biotechnologie, Philipps-Universität Marburg, Deutschhausstrasse 17A, D-35037 Marburg, Germany.