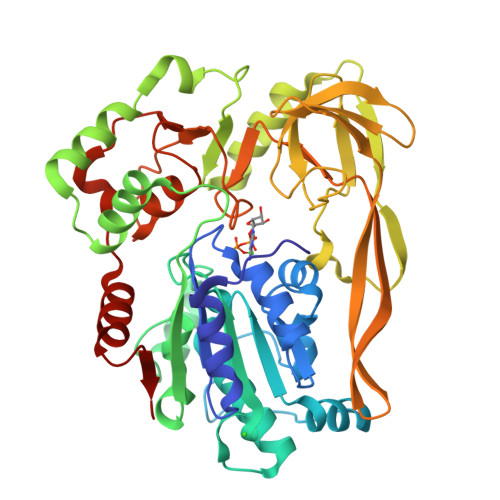
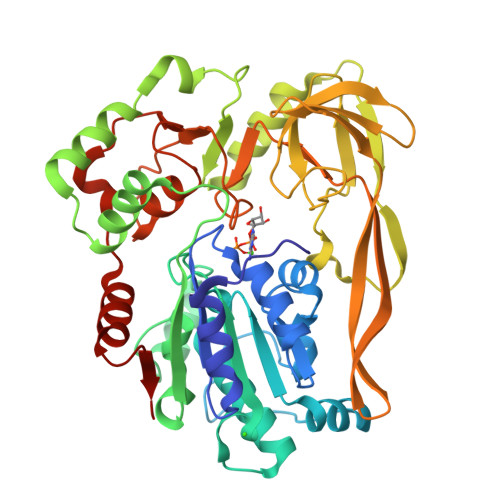
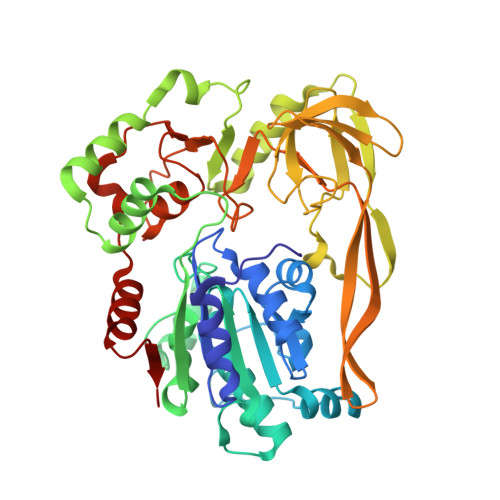
Crystal Structures of the Bspif1 Helicase Reveal that a Major Movement of the 2B SH3 Domain is Required for DNA Unwinding
Chen, W.-F., Dai, Y.-X., Duan, X.-L., Liu, N.-N., Shi, W., Li, N., Li, M., Dou, S.-X., Dong, Y.-H., Rety, S., Xi, X.-G.(2016) Nucleic Acids Res 44: 2949
- PubMed: 26809678
- DOI: https://doi.org/10.1093/nar/gkw033
- Primary Citation of Related Structures:
5FTB, 5FTC, 5FTD, 5FTE, 5FTF - PubMed Abstract:
Pif1 helicases are ubiquitous members of the SF1B family and are essential for maintaining genome stability. It was speculated that Pif1-specific motifs may fold in specific structures, conferring distinct activities upon it. Here, we report the crystal structures of the Pif1 helicase from Bacteroides spp with and without adenosine triphosphate (ATP) analog/ssDNA. BsPif1 shares structural similarities with RecD2 and Dda helicases but has specific features in the 1B and 2B domains. The highly conserved Pif1 family specific sequence motif interacts with and constraints a putative pin-loop in domain 1B in a precise conformation. More importantly, we found that the 2B domain which contains a specific extended hairpin undergoes a significant rotation and/or movement upon ATP and DNA binding, which is absolutely required for DNA unwinding. We therefore propose a mechanism for DNA unwinding in which the 2B domain plays a predominant role. The fact that the conformational change regulates Pif1 activity may provide insight into the puzzling observation that Pif1 becomes highly processive during break-induced replication in association with Polδ, while the isolated Pif1 has low processivity.
Organizational Affiliation:
College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, China.