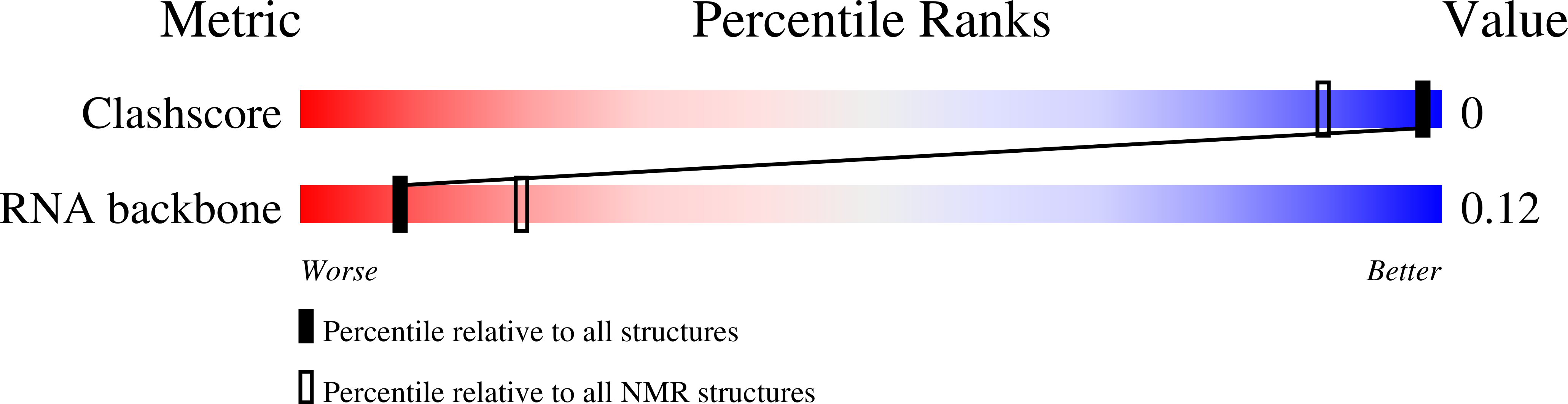Targeting RNA structure in SMN2 reverses spinal muscular atrophy molecular phenotypes.
Garcia-Lopez, A., Tessaro, F., Jonker, H.R.A., Wacker, A., Richter, C., Comte, A., Berntenis, N., Schmucki, R., Hatje, K., Petermann, O., Chiriano, G., Perozzo, R., Sciarra, D., Konieczny, P., Faustino, I., Fournet, G., Orozco, M., Artero, R., Metzger, F., Ebeling, M., Goekjian, P., Joseph, B., Schwalbe, H., Scapozza, L.(2018) Nat Commun 9: 2032-2032
- PubMed: 29795225
- DOI: https://doi.org/10.1038/s41467-018-04110-1
- Primary Citation of Related Structures:
5N5C - PubMed Abstract:
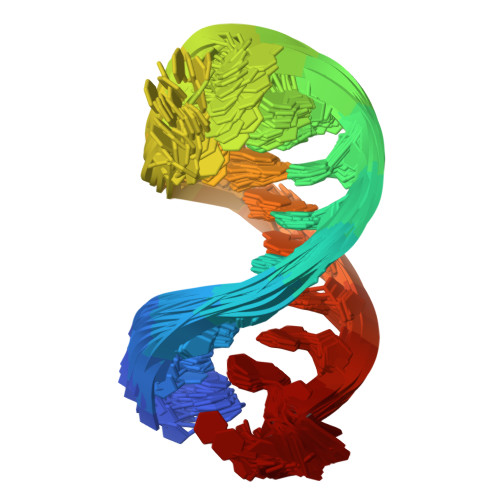
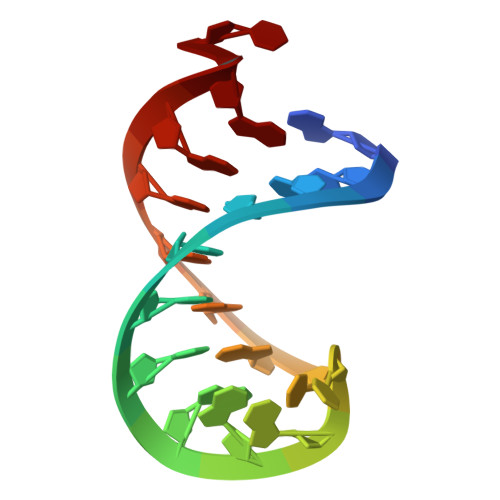
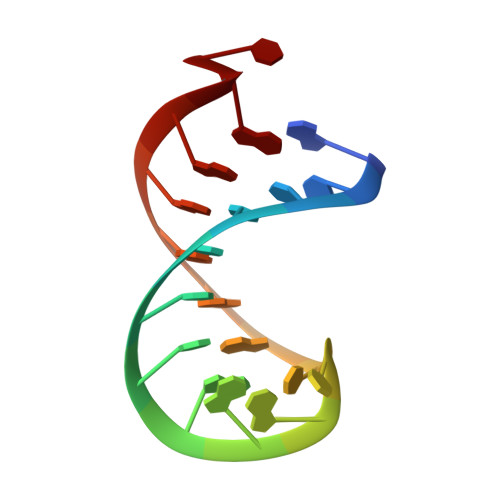
Modification of SMN2 exon 7 (E7) splicing is a validated therapeutic strategy against spinal muscular atrophy (SMA). However, a target-based approach to identify small-molecule E7 splicing modifiers has not been attempted, which could reveal novel therapies with improved mechanistic insight. Here, we chose as a target the stem-loop RNA structure TSL2, which overlaps with the 5' splicing site of E7. A small-molecule TSL2-binding compound, homocarbonyltopsentin (PK4C9), was identified that increases E7 splicing to therapeutic levels and rescues downstream molecular alterations in SMA cells. High-resolution NMR combined with molecular modelling revealed that PK4C9 binds to pentaloop conformations of TSL2 and promotes a shift to triloop conformations that display enhanced E7 splicing. Collectively, our study validates TSL2 as a target for small-molecule drug discovery in SMA, identifies a novel mechanism of action for an E7 splicing modifier, and sets a precedent for other splicing-mediated diseases where RNA structure could be similarly targeted.
Organizational Affiliation:
Pharmaceutical Biochemistry Group, School of Pharmaceutical Sciences, University of Lausanne and University of Geneva, Rue Michel-Servet 1, 1211, Geneva, Switzerland. amparo.garcialopez@unige.ch.