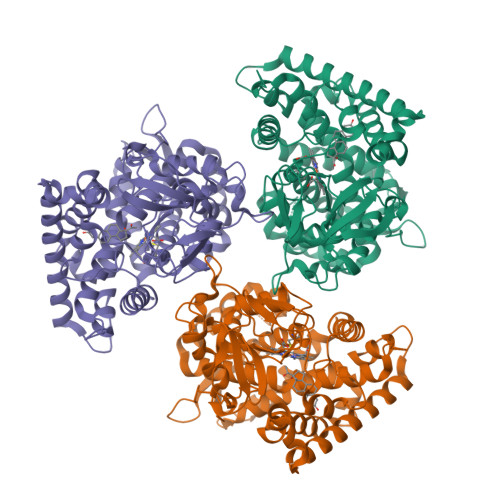
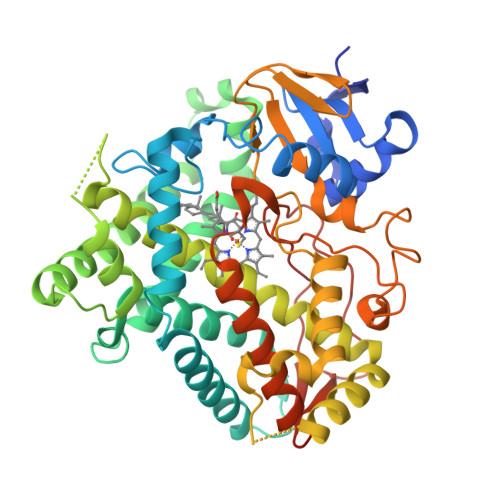
Functional analysis of human cytochrome P450 21A2 variants involved in congenital adrenal hyperplasia.
Wang, C., Pallan, P.S., Zhang, W., Lei, L., Yoshimoto, F.K., Waterman, M.R., Egli, M., Guengerich, F.P.(2017) J Biological Chem 292: 10767-10778
- PubMed: 28539365
- DOI: https://doi.org/10.1074/jbc.M117.792465
- Primary Citation of Related Structures:
5VBU - PubMed Abstract:
Cytochrome P450 (P450, CYP) 21A2 is the major steroid 21-hydroxylase, converting progesterone to 11-deoxycorticosterone and 17α-hydroxyprogesterone (17α-OH-progesterone) to 11-deoxycortisol. More than 100 CYP21A2 variants give rise to congenital adrenal hyperplasia (CAH). We previously reported a structure of WT human P450 21A2 with bound progesterone and now present a structure bound to the other substrate (17α-OH-progesterone). We found that the 17α-OH-progesterone- and progesterone-bound complex structures are highly similar, with only some minor differences in surface loop regions. Twelve P450 21A2 variants associated with either salt-wasting or nonclassical forms of CAH were expressed, purified, and analyzed. The catalytic activities of these 12 variants ranged from 0.00009% to 30% of WT P450 21A2 and the extent of heme incorporation from 10% to 95% of the WT. Substrate dissociation constants ( K s ) for four variants were 37-13,000-fold higher than for WT P450 21A2. Cytochrome b 5 , which augments several P450 activities, inhibited P450 21A2 activity. Similar to the WT enzyme, high noncompetitive intermolecular kinetic deuterium isotope effects (≥ 5.5) were observed for all six P450 21A2 variants examined for 21-hydroxylation of 21- d 3 -progesterone, indicating that C-H bond breaking is a rate-limiting step over a 10 4 -fold range of catalytic efficiency. Using UV-visible and CD spectroscopy, we found that P450 21A2 thermal stability assessed in bacterial cells and with purified enzymes differed among salt-wasting- and nonclassical-associated variants, but these differences did not correlate with catalytic activity. Our in-depth investigation of CAH-associated P450 21A2 variants reveals critical insight into the effects of disease-causing mutations on this important enzyme.
Organizational Affiliation:
From the Department of Biochemistry, Vanderbilt University School of Medicine, Nashville, Tennessee 37232-0146.