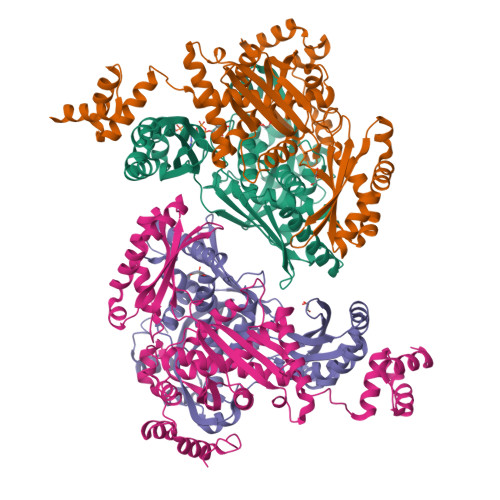
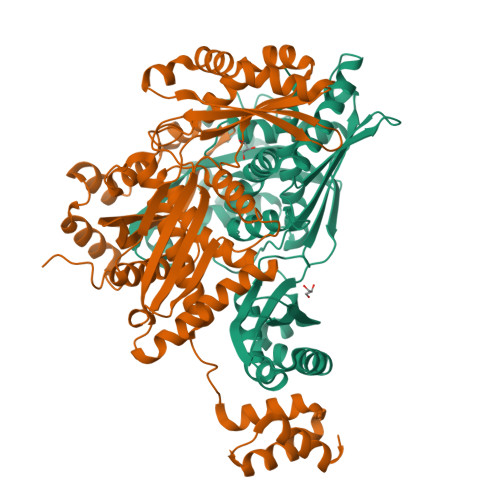
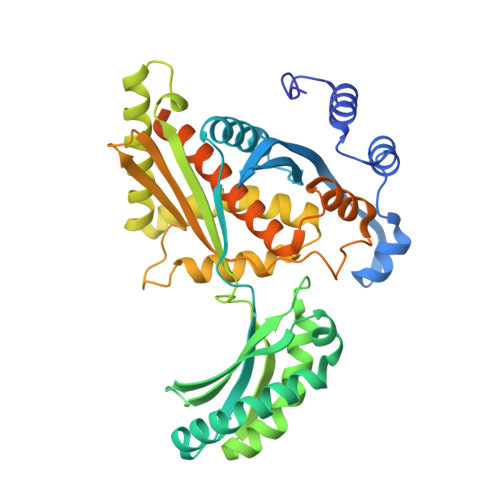
Structural Features and Domain Movements Controlling Substrate Binding and Cofactor Specificity in Class II HMG-CoA Reductase.
Miller, B.R., Kung, Y.(2018) Biochemistry 57: 654-662
- PubMed: 29224355
- DOI: https://doi.org/10.1021/acs.biochem.7b00999
- Primary Citation of Related Structures:
5WPJ, 5WPK - PubMed Abstract:
The key mevalonate pathway enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (HMGR) uses the cofactor NAD(P)H to reduce HMG-CoA to mevalonate in the production of countless metabolites and natural products. Although inhibition of HMGR by statin drugs is well-understood, several mechanistic details of HMGR catalysis remain unresolved, and the structural basis for the wide range of cofactor specificity for either NADH or NADPH among HMGRs from different organisms is also unknown. Here, we present crystal structures of HMGR from Streptococcus pneumoniae (SpHMGR) alongside kinetic data of the enzyme's cofactor preferences. Our structure of SpHMGR bound with its kinetically preferred NADPH cofactor suggests how NADPH-specific binding and recognition are achieved. In addition, our structure of HMG-CoA-bound SpHMGR reveals large, previously unknown conformational domain movements that may control HMGR substrate binding and enable cofactor exchange without intermediate release during the catalytic cycle. Taken together, this work provides critical new insights into both the HMGR reaction mechanism and the structural basis of cofactor specificity.
Organizational Affiliation:
Department of Chemistry, Bryn Mawr College , 101 North Merion Avenue, Bryn Mawr, Pennsylvania 19010, United States.