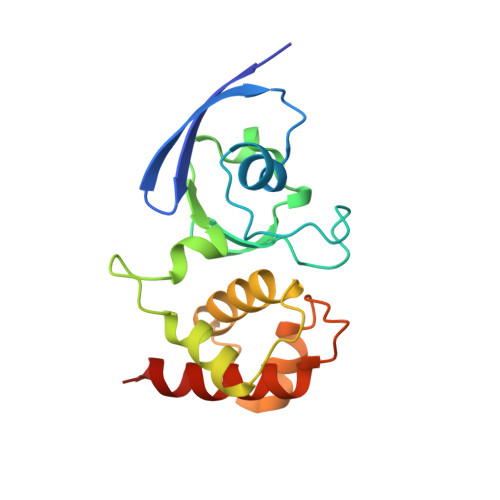
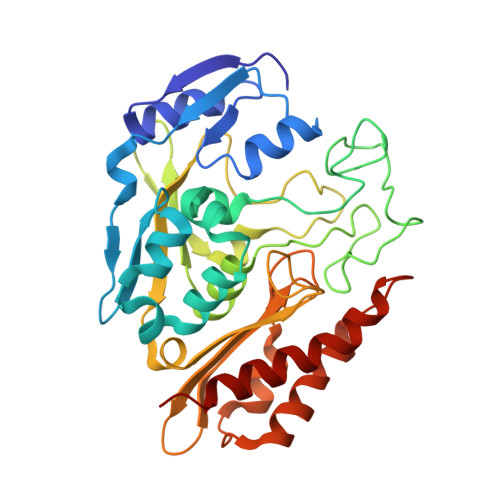
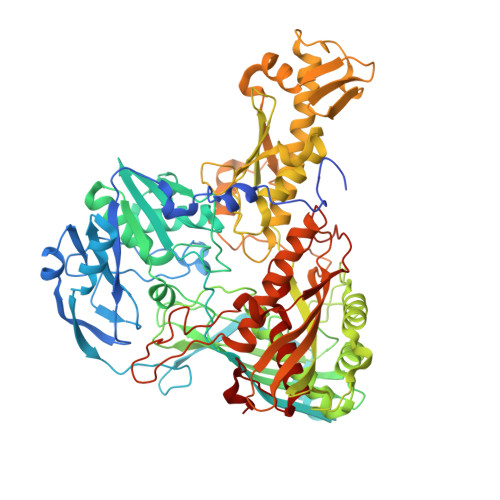
Crystal structure of an aldehyde oxidase from Methylobacillus sp. KY4400.
Uchida, H., Mikami, B., Yamane-Tanabe, A., Ito, A., Hirano, K., Oki, M.(2018) J Biochem 163: 321-328
- PubMed: 29319807
- DOI: https://doi.org/10.1093/jb/mvy004
- Primary Citation of Related Structures:
5Y6Q - PubMed Abstract:
Hetero-trimeric aldehyde oxidases of bacterial origin, which use O2 to catalyse the oxidation of various aldehydes but not those of aromatic N-heterocycles, belong to the xanthine oxidase family. In the present study, the crystal structure of a recombinant aldehyde oxidase from Methylobacillus sp. KY4400 (Mb-AOX) was determined at 2.5 Å resolution. The structures of its subunits resemble those of the corresponding subunits or domains of other structurally characterised enzymes belonging to the family, and include a [4Fe-4 S] cluster in the medium subunit like that found in Escherichia coli periplasmic aldehyde oxidoreductase (EP-AOR). A funnel leading to the si-face of the isoalloxazine ring of FAD, which is narrower than those in mouse liver AOX3 and human AOX1, is also present and it is even narrower than that in EP-AOR. The environment surrounding the ring in Mb-AOX and EP-AOR is subtly different, which might account for their different abilities to use O2. A remarkable characteristic of the Mo catalytic centre in Mb-AOX is a tryptophan situated near the centre instead of the alanine present in other xanthine oxidase family members. The tryptophan residue together with other residue differences might play an important role in binding to aldehydes such as n-heptylaldehyde in Mb-AOX.
Organizational Affiliation:
Department of Applied Chemistry and Biotechnology, Graduate School of Engineering, University of Fukui, 9-1, Bunkyo 3-Chome, Fukui 910-8507, Japan.