Redox Cofactor Rotates during Its Stepwise Decarboxylation: Molecular Mechanism of Conversion of Coproheme to Hemeb.
Milazzo, L., Gabler, T., Puhringer, D., Jandova, Z., Maresch, D., Michlits, H., Pfanzagl, V., Djinovic-Carugo, K., Oostenbrink, C., Furtmuller, P.G., Obinger, C., Smulevich, G., Hofbauer, S.(2019) ACS Catal 9: 6766-6782
- PubMed: 31423350
- DOI: https://doi.org/10.1021/acscatal.9b00963
- Primary Citation of Related Structures:
6FXJ, 6FXQ - PubMed Abstract:
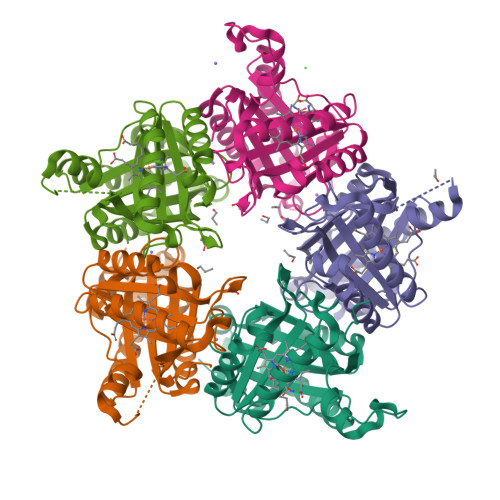
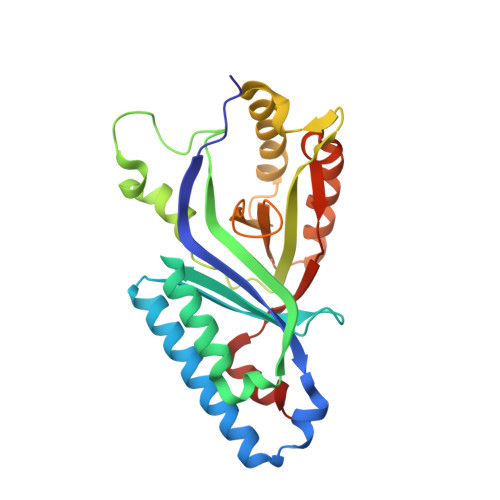
Coproheme decarboxylase (ChdC) catalyzes the last step in the heme biosynthesis pathway of monoderm bacteria with coproheme acting both as redox cofactor and substrate. Hydrogen peroxide mediates the stepwise decarboxylation of propionates 2 and 4 of coproheme. Here we present the crystal structures of coproheme-loaded ChdC from Listeria monocytogenes (LmChdC) and the three-propionate intermediate, for which the propionate at position 2 (p2) has been converted to a vinyl group and is rotated by 90° compared to the coproheme complex structure. Single, double, and triple mutants of LmChdC, in which H-bonding interactions to propionates 2, 4, 6, and 7 were eliminated, allowed us to obtain the assignment of the coproheme propionates by resonance Raman spectroscopy and to follow the H 2 O 2 -mediated conversion of coproheme to heme b . Substitution of H 2 O 2 by chlorite allowed us to monitor compound I formation in the inactive Y147H variant which lacks the catalytically essential Y147. This residue was demonstrated to be oxidized during turnover by using the spin-trap 2-methyl-2-nitrosopropane. Based on these findings and the data derived from molecular dynamics simulations of cofactor structures in distinct poses, we propose a reaction mechanism for the stepwise decarboxylation of coproheme that includes a 90° rotation of the intermediate three-propionate redox cofactor.
Organizational Affiliation:
Dipartimento di Chimica "Ugo Schiff", Università di Firenze, Via della Lastruccia 3-13, I-50019 Sesto Fiorentino (FI), Italy.