Discovery and Optimization of Chromone Derivatives as Novel Selective Phosphodiesterase 10 Inhibitors.
Yu, Y.F., Zhang, C., Huang, Y.Y., Zhang, S., Zhou, Q., Li, X., Lai, Z., Li, Z., Gao, Y., Wu, Y., Guo, L., Wu, D., Luo, H.B.(2020) ACS Chem Neurosci 11: 1058-1071
- PubMed: 32105440
- DOI: https://doi.org/10.1021/acschemneuro.0c00024
- Primary Citation of Related Structures:
6KO0, 6KO1 - PubMed Abstract:
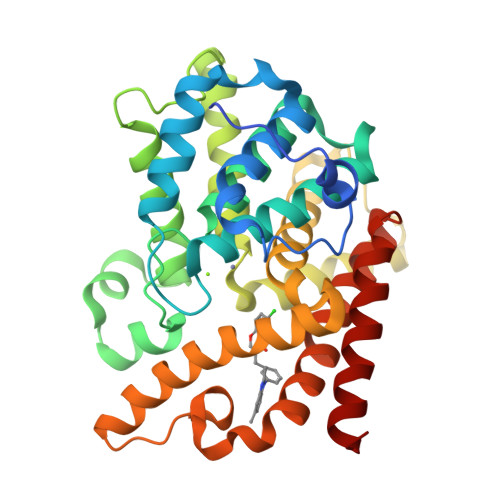
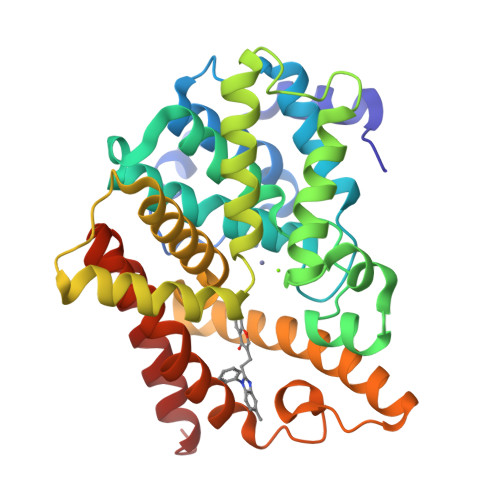
Phosphodiesterase 10 (PDE10) inhibitors have received much attention as promising therapeutic agents for central nervous system (CNS) disorders such as schizophrenia and Huntington's disease. Recently, a hit compound 1 with a novel chromone scaffold has shown moderate inhibitory activity against PDE10A (IC 50 = 500 nM). Hit-to-lead optimization has resulted in compound 3e with an improved inhibitory activity (IC 50 = 6.5 nM), remarkable selectivity (>95-fold over other PDEs), and good metabolic stability (RLM t 1/2 = 105 min) by using an integrated strategy (molecular modeling, chemical synthesis, bioassay, and cocrystal structure). The cocrystal structural information provides insights into the binding pattern of 3e in the PDE10A catalytic domain to highlight the key role of the halogen and hydrogen bonds toward Tyr524 and Tyr693, respectively, thereby resulting in high selectivity against other PDEs. These new observations are of benefit for the rational design of the next generation PDE10 inhibitors for CNS disorders.
Organizational Affiliation:
School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, China.