Crystal Structure of African Swine Fever Virus pS273R Protease and Implications for Inhibitor Design.
Li, G., Liu, X., Yang, M., Zhang, G., Wang, Z., Guo, K., Gao, Y., Jiao, P., Sun, J., Chen, C., Wang, H., Deng, W., Xiao, H., Li, S., Wu, H., Wang, Y., Cao, L., Jia, Z., Shang, L., Yang, C., Guo, Y., Rao, Z.(2020) J Virol 94
- PubMed: 32075933
- DOI: https://doi.org/10.1128/JVI.02125-19
- Primary Citation of Related Structures:
6LJ9, 6LJB - PubMed Abstract:
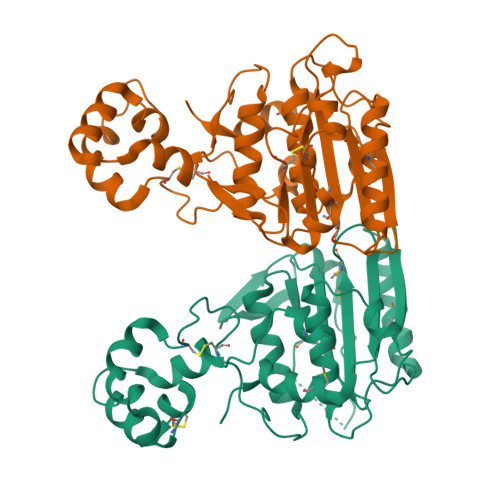
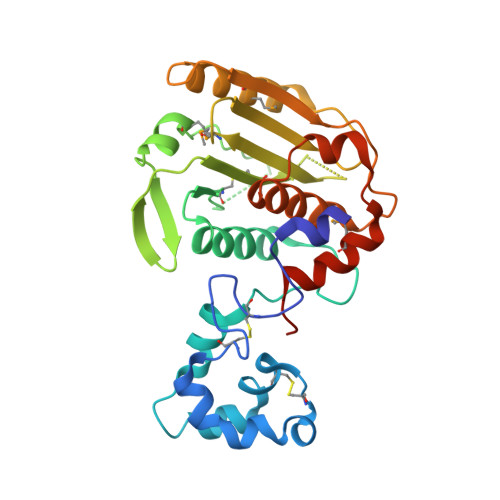
African swine fever (ASF) is a highly contagious hemorrhagic viral disease of domestic and wild pigs that is responsible for serious economic and production losses. It is caused by the African swine fever virus (ASFV), a large and complex icosahedral DNA virus of the Asfarviridae family. Currently, there is no effective treatment or approved vaccine against the ASFV. pS273R, a specific SUMO-1 cysteine protease, catalyzes the maturation of the pp220 and pp62 polyprotein precursors into core-shell proteins. Here, we present the crystal structure of the ASFV pS273R protease at a resolution of 2.3 Å. The overall structure of the pS273R protease is represented by two domains named the "core domain" and the N-terminal "arm domain." The "arm domain" contains the residues from M1 to N83, and the "core domain" contains the residues from N84 to A273. A structure analysis reveals that the "core domain" shares a high degree of structural similarity with chlamydial deubiquitinating enzyme, sentrin-specific protease, and adenovirus protease, while the "arm domain" is unique to ASFV. Further, experiments indicated that the "arm domain" plays an important role in maintaining the enzyme activity of ASFV pS273R. Moreover, based on the structural information of pS273R, we designed and synthesized several peptidomimetic aldehyde compounds at a submolar 50% inhibitory concentration, which paves the way for the design of inhibitors to target this severe pathogen. IMPORTANCE African swine fever virus, a large and complex icosahedral DNA virus, causes a deadly infection in domestic pigs. In addition to Africa and Europe, countries in Asia, including China, Vietnam, and Mongolia, were negatively affected by the hazards posed by ASFV outbreaks in 2018 and 2019, at which time more than 30 million pigs were culled. Until now, there has been no vaccine for protection against ASFV infection or effective treatments to cure ASF. Here, we solved the high-resolution crystal structure of the ASFV pS273R protease. The pS273R protease has a two-domain structure that distinguishes it from other members of the SUMO protease family, while the unique "arm domain" has been proven to be essential for its hydrolytic activity. Moreover, the peptidomimetic aldehyde compounds designed to target the substrate binding pocket exert prominent inhibitory effects and can thus be used in a potential lead for anti-ASFV drug development.
Organizational Affiliation:
State Key Laboratory of Medicinal Chemical Biology and College of Pharmacy, Nankai University, Tianjin, People's Republic of China.