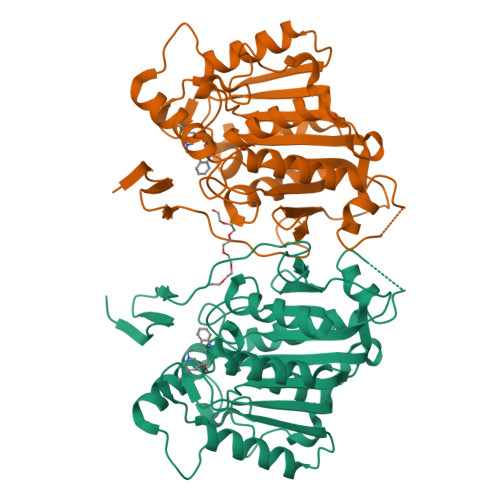
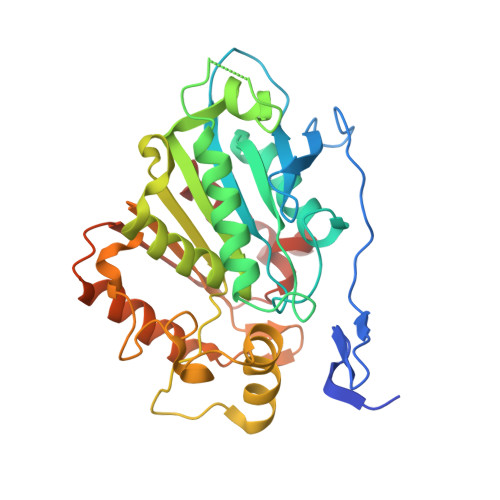
Structural basis of cycloaddition in biosynthesis of iboga and aspidosperma alkaloids.
Caputi, L., Franke, J., Bussey, K., Farrow, S.C., Vieira, I.J.C., Stevenson, C.E.M., Lawson, D.M., O'Connor, S.E.(2020) Nat Chem Biol 16: 383-386
- PubMed: 32066966
- DOI: https://doi.org/10.1038/s41589-019-0460-x
- Primary Citation of Related Structures:
6RJ8, 6RS4, 6RT8 - PubMed Abstract:
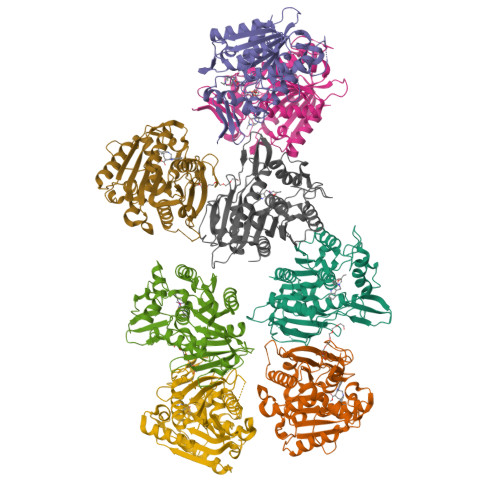
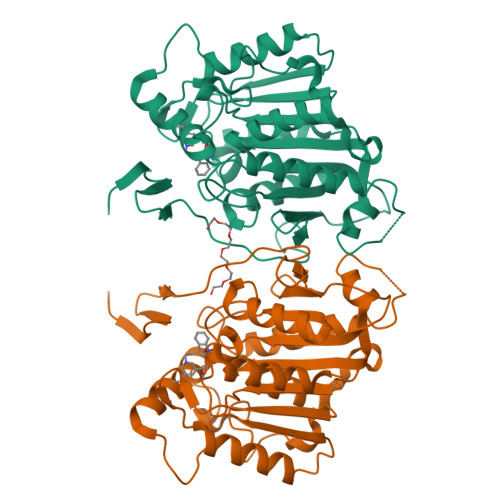
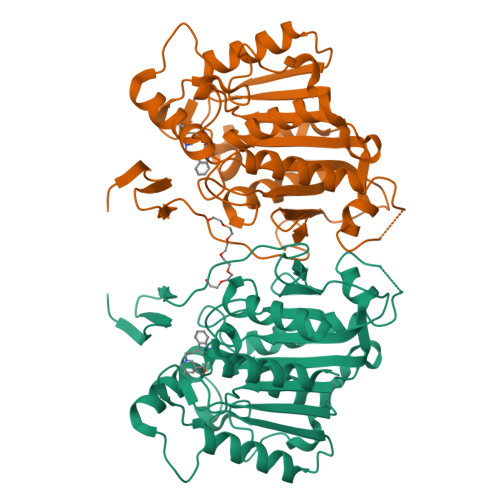
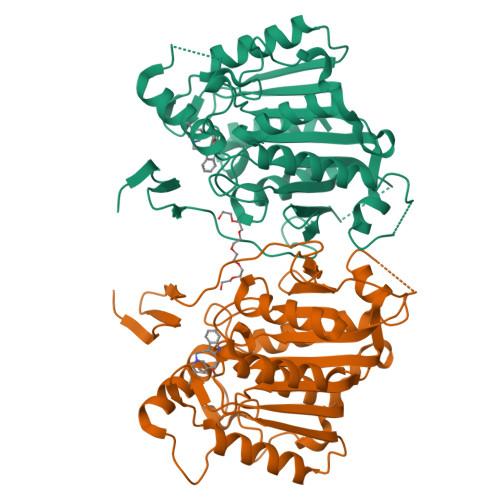
Cycloaddition reactions generate chemical complexity in a single step. Here we report the crystal structures of three homologous plant-derived cyclases involved in the biosynthesis of iboga and aspidosperma alkaloids. These enzymes act on the same substrate, named angryline, to generate three distinct scaffolds. Mutational analysis reveals how these highly similar enzymes control regio- and stereo-selectivity.
Organizational Affiliation:
Max Planck Institute of Chemical Ecology, Department of Natural Product Biosynthesis, Jena, Germany.