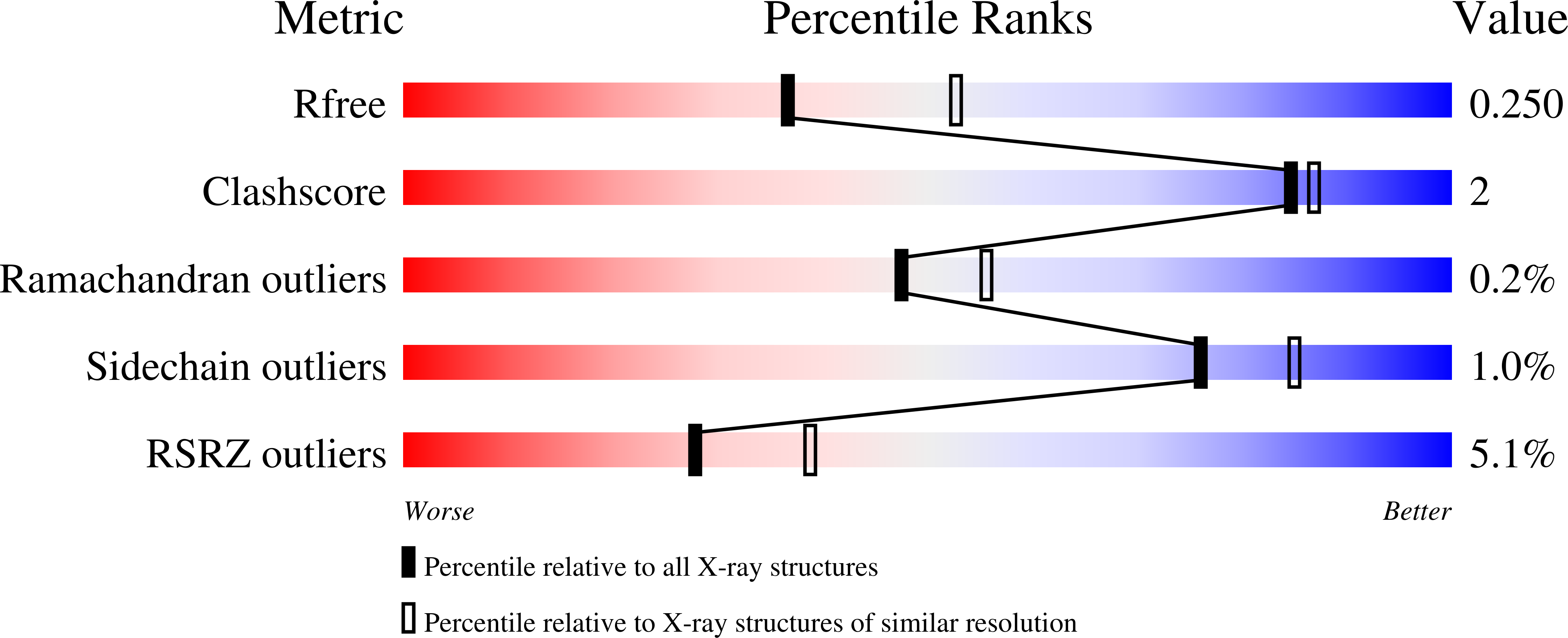
Structures of catalytic cycle intermediates of the Pyrococcus furiosus methionine adenosyltransferase demonstrate negative cooperativity in the archaeal orthologues.
Minici, C., Mosca, L., Ilisso, C.P., Cacciapuoti, G., Porcelli, M., Degano, M.(2020) J Struct Biol 210: 107462-107462
- PubMed: 31962159
- DOI: https://doi.org/10.1016/j.jsb.2020.107462
- Primary Citation of Related Structures:
6S81, 6S83 - PubMed Abstract:
Methionine adenosyltransferases catalyse the biosynthesis of S-adenosylmethionine, the primary methyl group donor in biochemical reactions, through the condensation of methionine and ATP. Here, we report the structural analysis of the Pyrococcus furiosus methionine adenosyltransferase (PfMAT) captured in the unliganded, substrate- and product-bound states. The conformational changes taking place during the enzymatic catalytic cycle are allosterically propagated by amino acid residues conserved in the archaeal orthologues to induce an asymmetric dimer structure. The distinct occupancy of the active sites within a PfMAT dimer is consistent with a half-site reactivity that is mediated by a product-induced negative cooperativity. The structures of intermediate states of PfMAT reported here suggest a distinct molecular mechanism for S-adenosylmethionine synthesis in Archaea, likely consequence of the evolutionary pressure to achieve protein stability under extreme conditions.
Organizational Affiliation:
Biocrystallography Unit, Division of Immunology, Transplantation, and Infectious Diseases, IRCCS Scientific Institute San Raffaele, 20132 Milan, Italy.