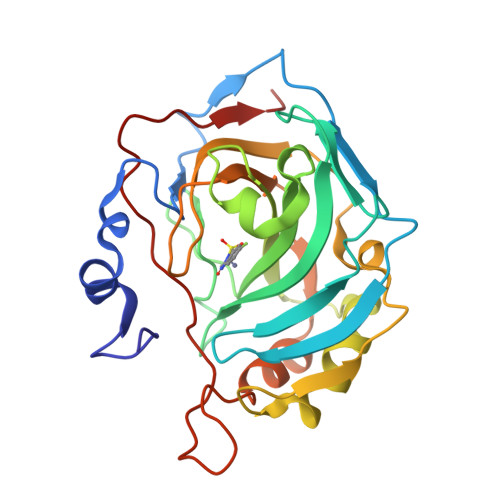
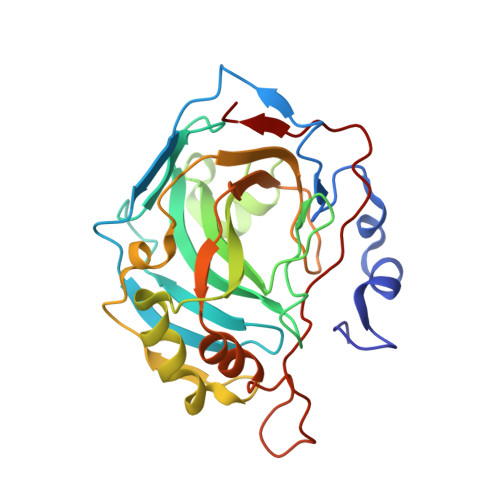
"A Sweet Combination": Developing Saccharin and Acesulfame K Structures for Selectively Targeting the Tumor-Associated Carbonic Anhydrases IX and XII.
Bua, S., Lomelino, C., Murray, A.B., Osman, S.M., ALOthman, Z.A., Bozdag, M., Abdel-Aziz, H.A., Eldehna, W.M., McKenna, R., Nocentini, A., Supuran, C.T.(2020) J Med Chem 63: 321-333
- PubMed: 31794211
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01669
- Primary Citation of Related Structures:
6U4Q, 6U4T, 6UGN, 6UGO, 6UGP, 6UGQ, 6UGR, 6UGZ, 6UH0 - PubMed Abstract:
The sweeteners saccharin ( SAC ) and acesulfame K ( ACE ) recently entered the topic of anticancer human carbonic anhydrase (CA, EC 4.2.1.1) inhibitors, as they showed to selectively inhibit the tumor-associated CAs IX/XII over ubiquitous CAs. A drug design strategy is here reported, which took SAC and ACE as leads and produced a series of 2 H -benzo[ e ][1,2,4]thiadiazin-3(4 H )-one-1,1-dioxides ( BTD ). Many derivatives showed greater potency ( K I s-CA IX 19.1-408.5 nM) and selectivity (II/IX SI 2-76) than the leads ( K I s-CA IX 103, 2400 nM; II/IX-SI 56, >4) against CA IX/XII over off-target isoforms. A thorough X-ray crystallographic study depicted their binding mode to both CA II and IX-mimic. The most representative BTDs were characterized in vitro for their antitumor activity against A549, PC-3, and HCT-116 cancer cell lines both in normoxia and hypoxia. The two most effective compounds were assayed for their effect on several apoptosis markers, identifying promising leads for the development of new anticancer drugs.
Organizational Affiliation:
Department of NEUROFARBA, Section of Pharmaceutical and Nutraceutical Sciences , University of Florence , via Ugo Schiff 6 , 50019 Sesto Fiorentino, Florence , Italy.