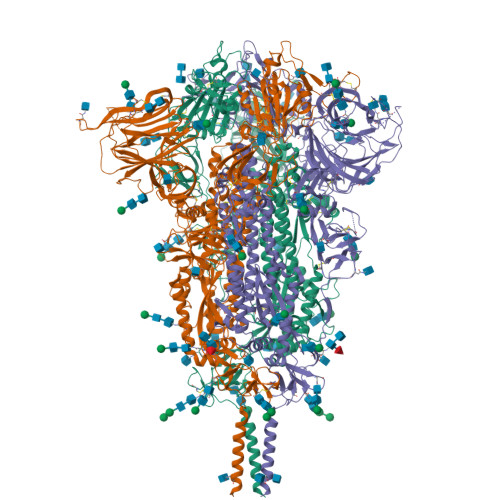
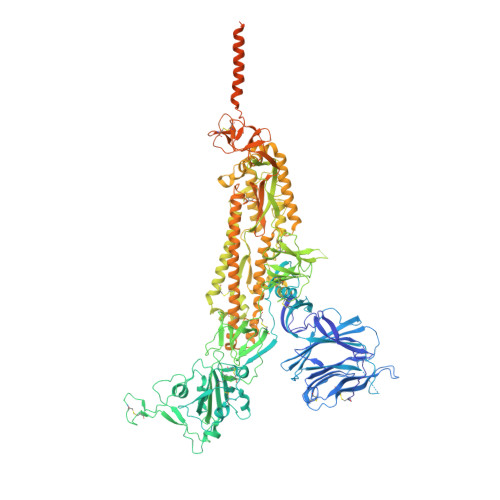
Distinct conformational states of SARS-CoV-2 spike protein.
Cai, Y., Zhang, J., Xiao, T., Peng, H., Sterling, S.M., Walsh Jr., R.M., Rawson, S., Rits-Volloch, S., Chen, B.(2020) Science 369: 1586-1592
- PubMed: 32694201
- DOI: https://doi.org/10.1126/science.abd4251
- Primary Citation of Related Structures:
6XR8, 6XRA - PubMed Abstract:
Intervention strategies are urgently needed to control the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. The trimeric viral spike (S) protein catalyzes fusion between viral and target cell membranes to initiate infection. Here, we report two cryo-electron microscopy structures derived from a preparation of the full-length S protein, representing its prefusion (2.9-angstrom resolution) and postfusion (3.0-angstrom resolution) conformations, respectively. The spontaneous transition to the postfusion state is independent of target cells. The prefusion trimer has three receptor-binding domains clamped down by a segment adjacent to the fusion peptide. The postfusion structure is strategically decorated by N-linked glycans, suggesting possible protective roles against host immune responses and harsh external conditions. These findings advance our understanding of SARS-CoV-2 entry and may guide the development of vaccines and therapeutics.
Organizational Affiliation:
Division of Molecular Medicine, Boston Children's Hospital, Boston, MA 02115, USA.