Analysis of SARS-CoV-2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species.
Wang, R., Zhang, Q., Ge, J., Ren, W., Zhang, R., Lan, J., Ju, B., Su, B., Yu, F., Chen, P., Liao, H., Feng, Y., Li, X., Shi, X., Zhang, Z., Zhang, F., Ding, Q., Zhang, T., Wang, X., Zhang, L.(2021) Immunity 54: 1611-1621.e5
- PubMed: 34166623
- DOI: https://doi.org/10.1016/j.immuni.2021.06.003
- Primary Citation of Related Structures:
7E8M - PubMed Abstract:
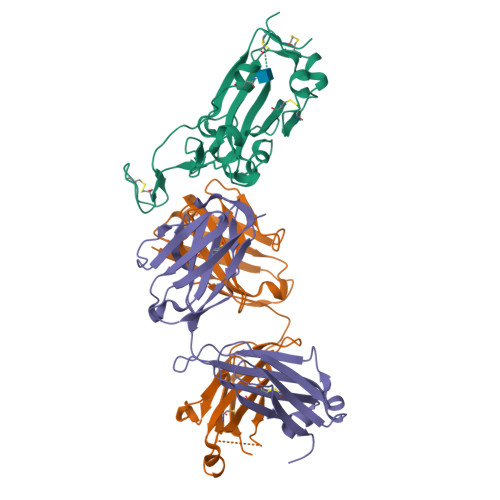
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants continue to emerge during the global pandemic and may facilitate escape from current antibody therapies and vaccine protection. Here we showed that the South African variant B.1.351 was the most resistant to current monoclonal antibodies and convalescent plasma from coronavirus disease 2019 (COVID-19)-infected individuals, followed by the Brazilian variant P.1 and the United Kingdom variant B.1.1.7. This resistance hierarchy corresponded with Y144del and 242-244del mutations in the N-terminal domain and K417N/T, E484K, and N501Y mutations in the receptor-binding domain (RBD) of SARS-CoV-2. Crystal structure analysis of the B.1.351 triple mutant (417N-484K-501Y) RBD complexed with the monoclonal antibody P2C-1F11 revealed the molecular basis for antibody neutralization and escape. B.1.351 and P.1 also acquired the ability to use mouse and mink ACE2 receptors for entry. Our results demonstrate major antigenic shifts and potential broadening of the host range for B.1.351 and P.1 variants, which poses serious challenges to current antibody therapies and vaccine protection.
Organizational Affiliation:
NexVac Research Center, Comprehensive AIDS Research Center, Center for Infectious Disease Research, Beijing Advanced Innovation Center for Structural Biology, School of Medicine, Tsinghua University, Beijing 100084, China; Tsinghua-Peking Joint Center for Life Sciences, Beijing 100084, China.