LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants.
Westendorf, K., Wang, L., Zentelis, S., Foster, D., Vaillancourt, P., Wiggin, M., Lovett, E., van der Lee, R., Hendle, J., Pustilnik, A., Sauder, J.M., Kraft, L., Hwang, Y., Siegel, R.W., Chen, J., Heinz, B.A., Higgs, R.E., Kallewaard, N., Jepson, K., Goya, R., Smith, M.A., Collins, D.W., Pellacani, D., Xiang, P., de Puyraimond, V., Ricicova, M., Devorkin, L., Pritchard, C., O'Neill, A., Dalal, K., Panwar, P., Dhupar, H., Garces, F.A., Cohen, C., Dye, J., Huie, K.E., Badger, C.V., Kobasa, D., Audet, J., Freitas, J.J., Hassanali, S., Hughes, I., Munoz, L., Palma, H.C., Ramamurthy, B., Cross, R.W., Geisbert, T.W., Menacherry, V., Lokugamage, K., Borisevich, V., Lanz, I., Anderson, L., Sipahimalani, P., Corbett, K.S., Yang, E.S., Zhang, Y., Shi, W., Zhou, T., Choe, M., Misasi, J., Kwong, P.D., Sullivan, N.J., Graham, B.S., Fernandez, T.L., Hansen, C.L., Falconer, E., Mascola, J.R., Jones, B.E., Barnhart, B.C.(2022) bioRxiv
- PubMed: 33972947
- DOI: https://doi.org/10.1101/2021.04.30.442182
- Primary Citation of Related Structures:
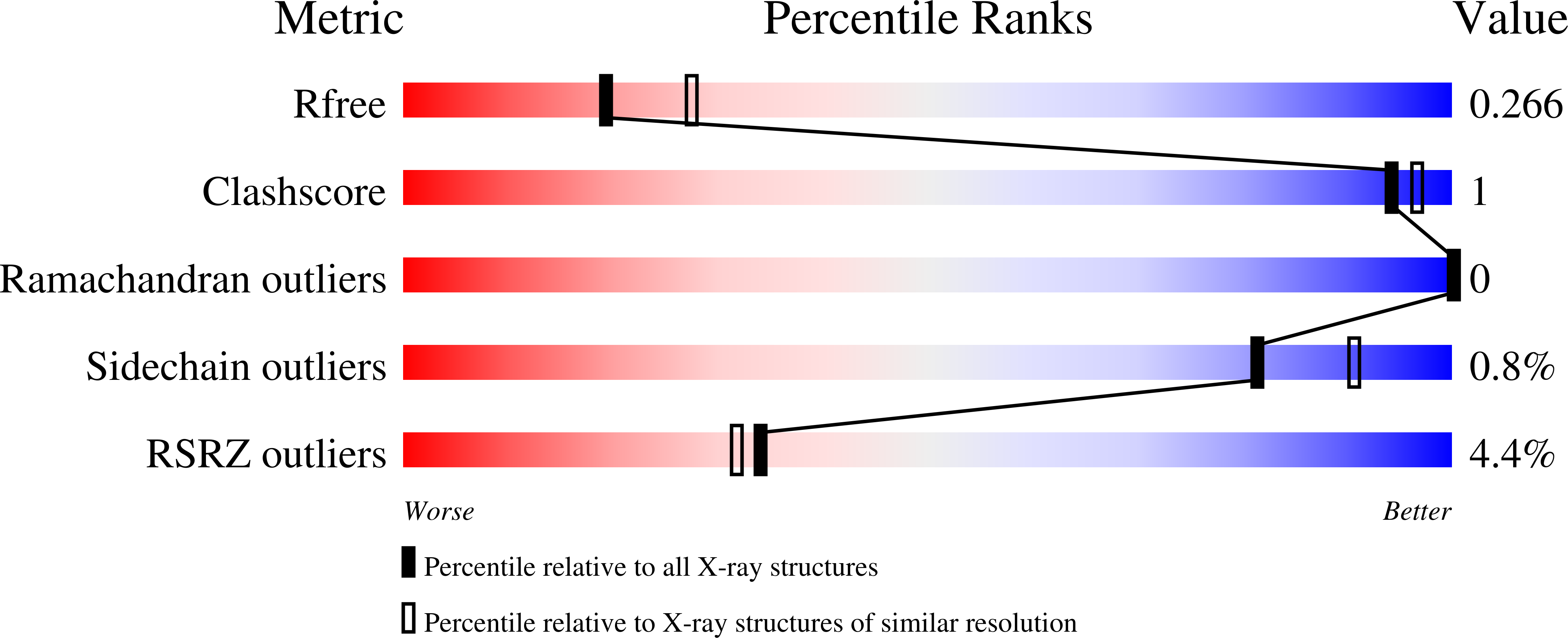
7MMO - PubMed Abstract:
SARS-CoV-2 neutralizing monoclonal antibodies (mAbs) can reduce the risk of hospitalization when administered early during COVID-19 disease. However, the emergence of variants of concern has negatively impacted the therapeutic use of some authorized mAbs. Using a high throughput B-cell screening pipeline, we isolated a highly potent SARS-CoV-2 spike glycoprotein receptor binding domain (RBD)-specific antibody called LY-CoV1404 (also known as bebtelovimab). LY-CoV1404 potently neutralizes authentic SARS-CoV-2 virus, including the prototype, B.1.1.7, B.1.351 and B.1.617.2). In pseudovirus neutralization studies, LY-CoV1404 retains potent neutralizing activity against numerous variants including B.1.1.7, B.1.351, B.1.617.2, B.1.427/B.1.429, P.1, B.1.526, B.1.1.529, and the BA.2 subvariant and retains binding to spike proteins with a variety of underlying RBD mutations including K417N, L452R, E484K, and N501Y. Structural analysis reveals that the contact residues of the LY-CoV1404 epitope are highly conserved with the exception of N439 and N501. Notably, the binding and neutralizing activity of LY-CoV1404 is unaffected by the most common mutations at these positions (N439K and N501Y). The breadth of reactivity to amino acid substitutions present among current VOC together with broad and potent neutralizing activity and the relatively conserved epitope suggest that LY-CoV1404 has the potential to be an effective therapeutic agent to treat all known variants causing COVID-19. LY-CoV1404 is a potent SARS-CoV-2-binding antibody that neutralizes all known variants of concern and whose epitope is rarely mutated. LY-CoV1404 potently neutralizes SARS-CoV-2 authentic virus and known variants of concern including the B.1.1.529 (Omicron), the BA.2 Omicron subvariant, and B.1.617.2 (Delta) variantsNo loss of potency against currently circulating variantsBinding epitope on RBD of SARS-CoV-2 is rarely mutated in GISAID databaseBreadth of neutralizing activity and potency supports clinical development.