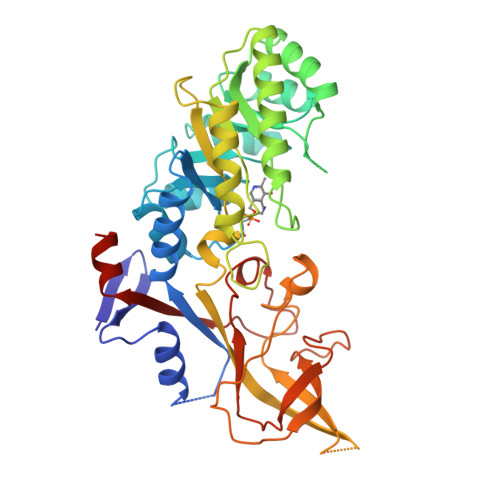
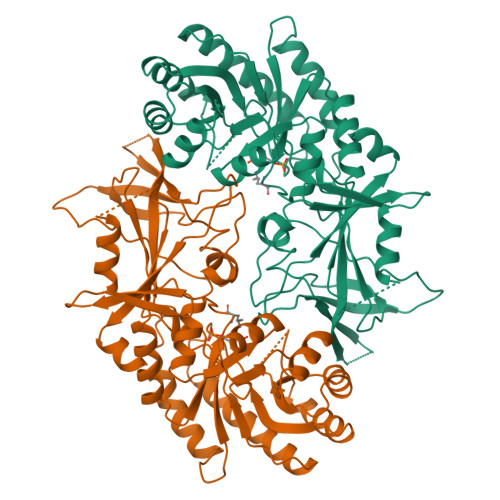
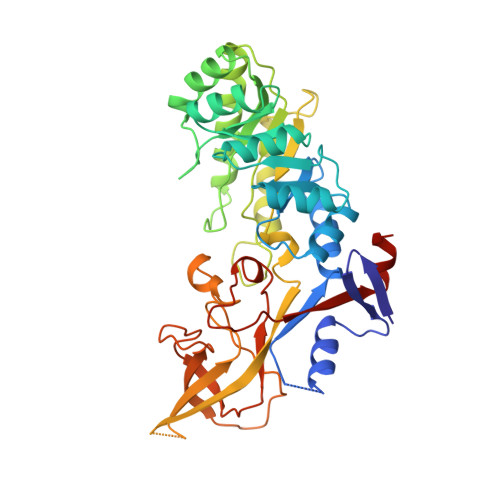
Structure of mammalian ornithine decarboxylase at 1.6 A resolution: stereochemical implications of PLP-dependent amino acid decarboxylases.
Kern, A.D., Oliveira, M.A., Coffino, P., Hackert, M.L.(1999) Structure 7: 567-581
- PubMed: 10378276
- DOI: https://doi.org/10.1016/s0969-2126(99)80073-2
- Primary Citation of Related Structures:
7ODC - PubMed Abstract:
Pyridoxal-5'-phosphate (PLP) dependent enzymes catalyze a broad range of reactions, resulting in bond cleavage at C alpha, C beta, or C gamma carbons of D and L amino acid substrates. Ornithine decarboxylase (ODC) is a PLP-dependent enzyme that controls a critical step in the biosynthesis of polyamines, small organic polycations whose controlled levels are essential for proper growth. ODC inhibition has applications for the treatment of certain cancers and parasitic ailments such as African sleeping sickness. The structure of truncated mouse ODC (mODC') was determined by multiple isomorphous replacement methods and refined to 1.6 A resolution. This is the first structure of a Group IV decarboxylase. The monomer contains two domains: an alpha/beta barrel that binds the cofactor, and a second domain consisting mostly of beta structure. Only the dimer is catalytically active, as the active sites are constructed of residues from both monomers. The interactions stabilizing the dimer shed light on its regulation by antizyme. The overall structure and the environment of the cofactor are compared with those of alanine racemase. The analysis of the mODC' structure and its comparison with alanine racemase, together with modeling studies of the external aldimine intermediate, provide insight into the stereochemical characteristics of PLP-dependent decarboxylation. The structure comparison reveals stereochemical differences with other PLP-dependent enzymes and the bacterial ODC. These characteristics may be exploited in the design of new inhibitors specific for eukaryotic and bacterial ODCs, and provide the basis for a detailed understanding of the mechanism by which these enzymes regulate reaction specificity.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Texas at Austin 78712, USA.