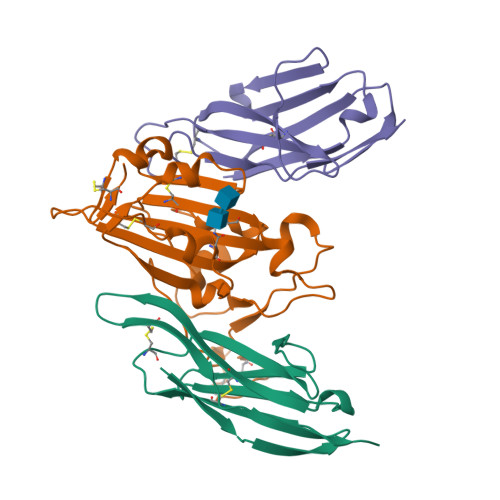
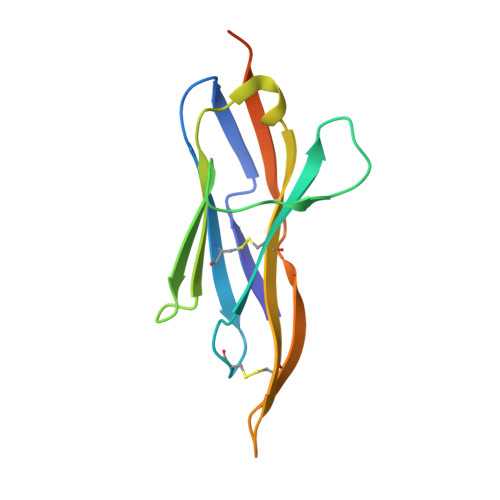
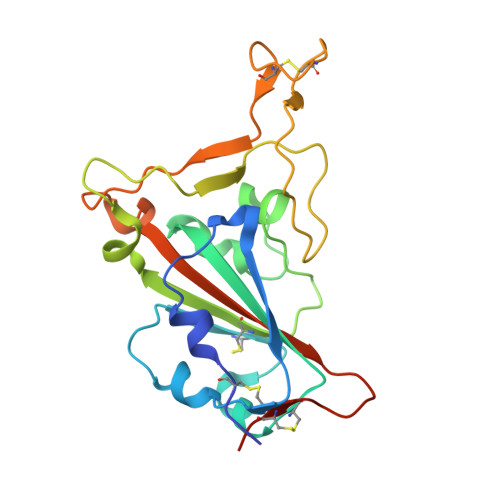
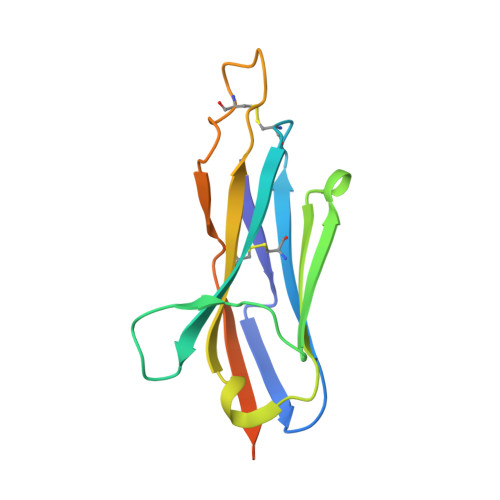
Shark nanobodies with potent SARS-CoV-2 neutralizing activity and broad sarbecovirus reactivity.
Chen, W.H., Hajduczki, A., Martinez, E.J., Bai, H., Matz, H., Hill, T.M., Lewitus, E., Chang, W.C., Dawit, L., Peterson, C.E., Rees, P.A., Ajayi, A.B., Golub, E.S., Swafford, I., Dussupt, V., David, S., Mayer, S.V., Soman, S., Kuklis, C., Corbitt, C., King, J., Choe, M., Sankhala, R.S., Thomas, P.V., Zemil, M., Wieczorek, L., Hart, T., Duso, D., Kummer, L., Yan, L., Sterling, S.L., Laing, E.D., Broder, C.C., Williams, J.K., Davidson, E., Doranz, B.J., Krebs, S.J., Polonis, V.R., Paquin-Proulx, D., Rolland, M., Reiley, W.W., Gromowski, G.D., Modjarrad, K., Dooley, H., Joyce, M.G.(2023) Nat Commun 14: 580-580
- PubMed: 36737435
- DOI: https://doi.org/10.1038/s41467-023-36106-x
- Primary Citation of Related Structures:
7S83 - PubMed Abstract:
Despite rapid and ongoing vaccine and therapeutic development, SARS-CoV-2 continues to evolve and evade, presenting a need for next-generation diverse therapeutic modalities. Here we show that nurse sharks immunized with SARS-CoV-2 recombinant receptor binding domain (RBD), RBD-ferritin (RFN), or spike protein ferritin nanoparticle (SpFN) immunogens elicit a set of new antigen receptor antibody (IgNAR) molecules that target two non-overlapping conserved epitopes on the spike RBD. Representative shark antibody variable NAR-Fc chimeras (ShAbs) targeting either of the two epitopes mediate cell-effector functions, with high affinity to all SARS-CoV-2 viral variants of concern, including the divergent Omicron strains. The ShAbs potently cross-neutralize SARS-CoV-2 WA-1, Alpha, Beta, Delta, Omicron BA.1 and BA.5, and SARS-CoV-1 pseudoviruses, and confer protection against SARS-CoV-2 challenge in the K18-hACE2 transgenic mouse model. Structural definition of the RBD-ShAb01-ShAb02 complex enabled design and production of multi-specific nanobodies with enhanced neutralization capacity, and picomolar affinity to divergent sarbecovirus clade 1a, 1b and 2 RBD molecules. These shark nanobodies represent potent immunotherapeutics both for current use, and future sarbecovirus pandemic preparation.
Organizational Affiliation:
Emerging Infectious Diseases Branch, Walter Reed Army Institute of Research, Silver Spring, MD, USA.