Unmasking the Conformational Stability and Inhibitor Binding to SARS-CoV-2 Main Protease Active Site Mutants and Miniprecursor.
Kovalevsky, A., Coates, L., Kneller, D.W., Ghirlando, R., Aniana, A., Nashed, N.T., Louis, J.M.(2022) J Mol Biology 434: 167876-167876
- PubMed: 36334779
- DOI: https://doi.org/10.1016/j.jmb.2022.167876
- Primary Citation of Related Structures:
8E4J, 8E4R - PubMed Abstract:
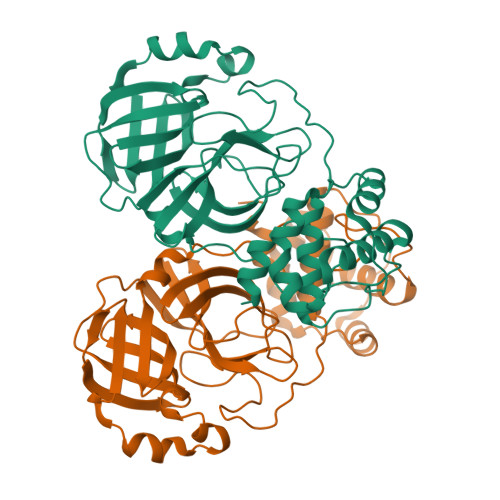
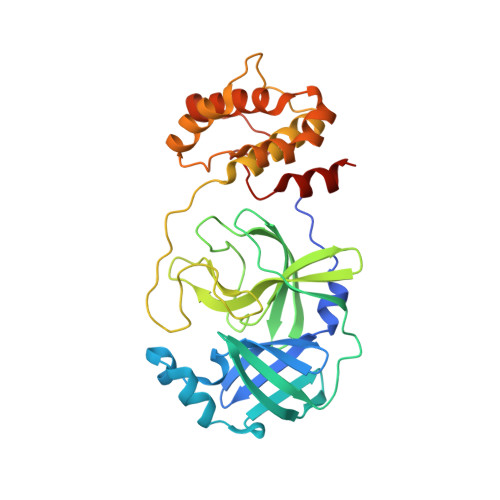
We recently demonstrated that inhibitor binding reorganizes the oxyanion loop of a monomeric catalytic domain of SARS CoV-2 main protease (MPro) from an unwound (E) to a wound (active, E*) conformation, independent of dimerization. Here we assess the effect of the flanking N-terminal residues, to imitate the MPro precursor prior to its autoprocessing, on conformational equilibria rendering stability and inhibitor binding. Thermal denaturation (T m ) of C145A mutant, unlike H41A, increases by 6.8 °C, relative to wild-type mature dimer. An inactivating H41A mutation to maintain a miniprecursor containing TSAVL[Q or E] of the flanking nsp4 sequence in an intact form [ (-6) MPro H41A and (-6*) MPro H41A , respectively], and its corresponding mature MPro H41A were systematically examined. While the H41A mutation exerts negligible effect on T m and dimer dissociation constant (K dimer ) of MPro H41A , relative to the wild type MPro, both miniprecursors show a 4-5 °C decrease in T m and > 85-fold increase in K dimer as compared to MPro H41A . The K d for the binding of the covalent inhibitor GC373 to (-6*) MPro H41A increases ∼12-fold, relative to MPro H41A , concomitant with its dimerization. While the inhibitor-free dimer exhibits a state in transit from E to E* with a conformational asymmetry of the protomers' oxyanion loops and helical domains, inhibitor binding restores the asymmetry to mature-like oxyanion loop conformations (E*) but not of the helical domains. Disorder of the terminal residues 1-2 and 302-306 observed in both structures suggest that N-terminal autoprocessing is tightly coupled to the E-E* equilibrium and stable dimer formation.
Organizational Affiliation:
Neutron Scattering Division, Oak Ridge National Laboratory, 1 Bethel Valley Road, Oak Ridge, TN 37831, USA. Electronic address: kovalevskyay@ornl.gov.