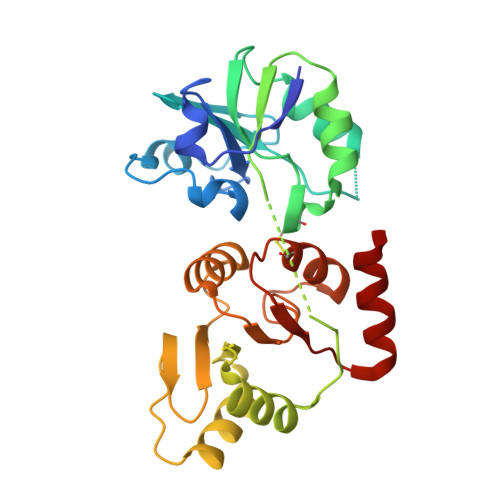
Identification of the SARS-unique domain of SARS-CoV-2 as an antiviral target.
Qin, B., Li, Z., Tang, K., Wang, T., Xie, Y., Aumonier, S., Wang, M., Yuan, S., Cui, S.(2023) Nat Commun 14: 3999-3999
- PubMed: 37414753
- DOI: https://doi.org/10.1038/s41467-023-39709-6
- Primary Citation of Related Structures:
8GQC, 8HBL - PubMed Abstract:
SARS-CoV-2 nsp3 is essential for viral replication and host responses. The SARS-unique domain (SUD) of nsp3 exerts its function through binding to viral and host proteins and RNAs. Herein, we show that SARS-CoV-2 SUD is highly flexible in solution. The intramolecular disulfide bond of SARS-CoV SUD is absent in SARS-CoV-2 SUD. Incorporating this bond in SARS-CoV-2 SUD allowed crystal structure determination to 1.35 Å resolution. However, introducing this bond in SARS-CoV-2 genome was lethal for the virus. Using biolayer interferometry, we screened compounds directly binding to SARS-CoV-2 SUD and identified theaflavin 3,3'-digallate (TF3) as a potent binder, K d 2.8 µM. TF3 disrupted the SUD-guanine quadruplex interactions and exhibited anti-SARS-CoV-2 activity in Vero E6-TMPRSS2 cells with an EC 50 of 5.9 µM and CC 50 of 98.5 µM. In this work, we provide evidence that SARS-CoV-2 SUD harbors druggable sites for antiviral development.
Organizational Affiliation:
NHC Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology Chinese Academy of Medical Sciences & Peking Union Medical College, 100730, Beijing, China.