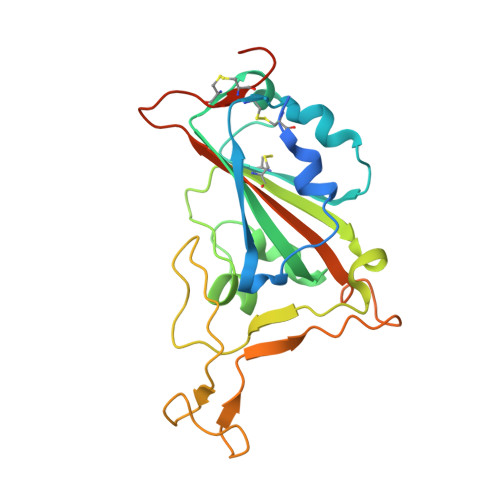
Cross-species recognition of bat coronavirus RsYN04 and cross-reaction of SARS-CoV-2 antibodies against the virus.
Zhao, R., Niu, S., Han, P., Gao, Y., Liu, D., Luo, C., Liu, H., Liu, B., Xu, Y., Qi, J., Chen, Z., Shi, W., Wu, L., Gao, G.F., Wang, Q.(2023) Zool Res 44: 1015-1025
- PubMed: 37804113
- DOI: https://doi.org/10.24272/j.issn.2095-8137.2023.187
- Primary Citation of Related Structures:
8J5J - PubMed Abstract:
Following the outbreak of coronavirus disease 2019 (COVID-19), several severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related coronaviruses have been discovered. Previous research has identified a novel lineage of SARS-CoV-2-related CoVs in bats, including RsYN04, which recognizes human angiotensin-converting enzyme 2 (ACE2) and thus poses a potential threat to humans. Here, we screened the binding of the RsYN04 receptor-binding domain (RBD) to ACE2 orthologs from 52 animal species and found that the virus showed a narrower ACE2-binding spectrum than SARS-CoV-2. However, the presence of the T484W mutation in the RsYN04 RBD broadened its range. We also evaluated 44 SARS-CoV-2 antibodies targeting seven epitope communities in the SARS-CoV-2 RBD, together with serum obtained from COVID-19 convalescents and vaccinees, to determine their cross-reaction against RsYN04. Results showed that no antibodies, except for the RBD-6 and RBD-7 classes, bound to the RsYN04 RBD, indicating substantial immune differences from SARS-CoV-2. Furthermore, the structure of the RsYN04 RBD in complex with cross-reactive antibody S43 in RBD-7 revealed a potently broad epitope for the development of therapeutics and vaccines. Our findings suggest RsYN04 and other viruses belonging to the same clade have the potential to infect several species, including humans, highlighting the necessity for viral surveillance and development of broad anti-coronavirus countermeasures.
Organizational Affiliation:
Institute of Physical Science and Information, Anhui University, Hefei, Anhui 230039, China.