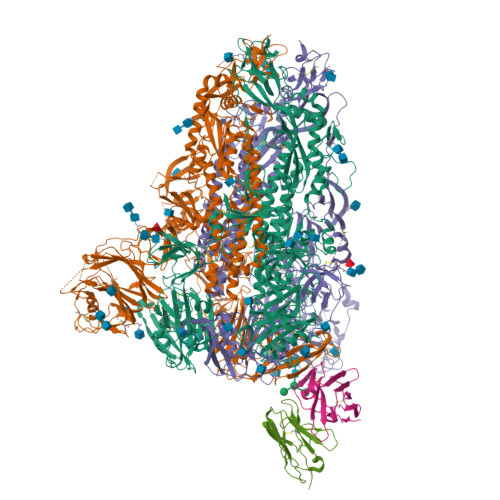
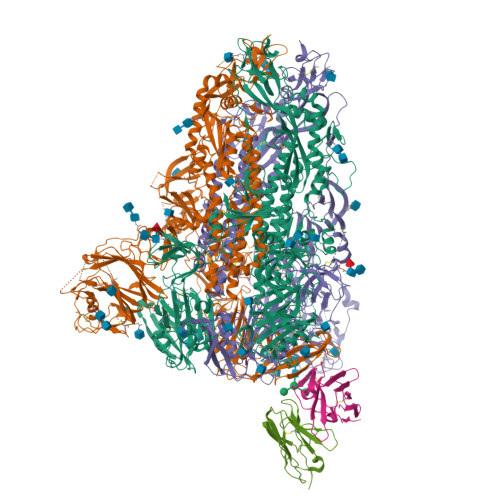
A MERS-CoV antibody neutralizes a pre-emerging group 2c bat coronavirus.
Tse, L.V., Hou, Y.J., McFadden, E., Lee, R.E., Scobey, T.D., Leist, S.R., Martinez, D.R., Meganck, R.M., Schafer, A., Yount, B.L., Mascenik, T., Powers, J.M., Randell, S.H., Zhang, Y., Wang, L., Mascola, J., McLellan, J.S., Baric, R.S.(2023) Sci Transl Med 15: eadg5567-eadg5567
- PubMed: 37756379
- DOI: https://doi.org/10.1126/scitranslmed.adg5567
- Primary Citation of Related Structures:
8SAK - PubMed Abstract:
The repeated emergence of zoonotic human betacoronaviruses (β-CoVs) dictates the need for broad therapeutics and conserved epitope targets for countermeasure design. Middle East respiratory syndrome (MERS)-related coronaviruses (CoVs) remain a pressing concern for global health preparedness. Using metagenomic sequence data and CoV reverse genetics, we recovered a full-length wild-type MERS-like BtCoV/ li /GD/2014-422 (BtCoV-422) recombinant virus, as well as two reporter viruses, and evaluated their human emergence potential and susceptibility to currently available countermeasures. Similar to MERS-CoV, BtCoV-422 efficiently used human and other mammalian dipeptidyl peptidase protein 4 (DPP4) proteins as entry receptors and an alternative DPP4-independent infection route in the presence of exogenous proteases. BtCoV-422 also replicated efficiently in primary human airway, lung endothelial, and fibroblast cells, although less efficiently than MERS-CoV. However, BtCoV-422 shows minor signs of infection in 288/330 human DPP4 transgenic mice. Several broad CoV antivirals, including nucleoside analogs and 3C-like/M pro protease inhibitors, demonstrated potent inhibition against BtCoV-422 in vitro. Serum from mice that received a MERS-CoV mRNA vaccine showed reduced neutralizing activity against BtCoV-422. Although most MERS-CoV-neutralizing monoclonal antibodies (mAbs) had limited activity, one anti-MERS receptor binding domain mAb, JC57-11, neutralized BtCoV-422 potently. A cryo-electron microscopy structure of JC57-11 in complex with BtCoV-422 spike protein revealed the mechanism of cross-neutralization involving occlusion of the DPP4 binding site, highlighting its potential as a broadly neutralizing mAb for group 2c CoVs that use DPP4 as a receptor. These studies provide critical insights into MERS-like CoVs and provide candidates for countermeasure development.
Organizational Affiliation:
Department of Molecular Microbiology and Immunology, Saint Louis University, St. Louis, MO 63014, USA.