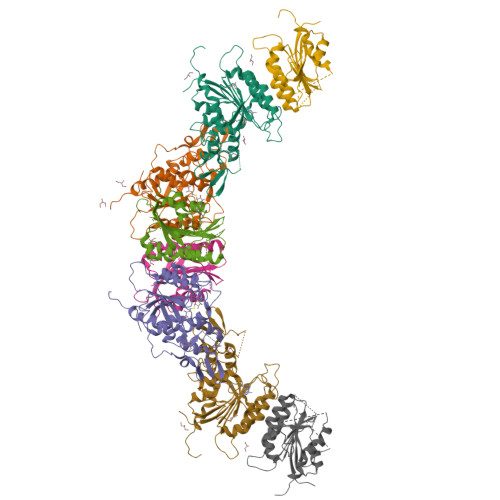
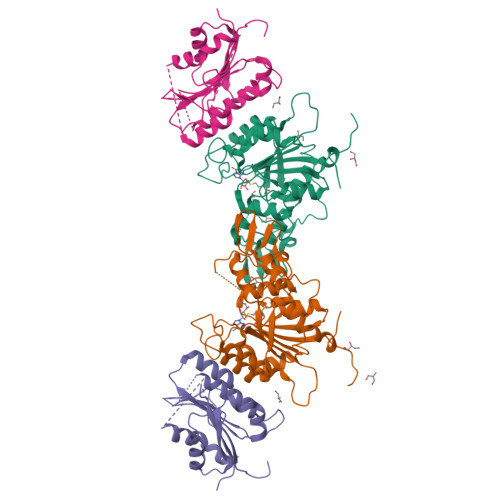
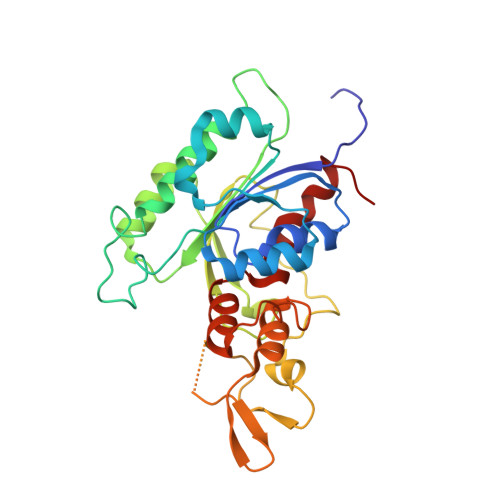
Structure-guided functional suppression of AML-associated DNMT3A hotspot mutations.
Lu, J., Guo, Y., Yin, J., Chen, J., Wang, Y., Wang, G.G., Song, J.(2024) Nat Commun 15: 3111-3111
- PubMed: 38600075
- DOI: https://doi.org/10.1038/s41467-024-47398-y
- Primary Citation of Related Structures:
8TDR, 8TE1, 8TE3, 8TE4 - PubMed Abstract:
DNA methyltransferases DNMT3A- and DNMT3B-mediated DNA methylation critically regulate epigenomic and transcriptomic patterning during development. The hotspot DNMT3A mutations at the site of Arg822 (R882) promote polymerization, leading to aberrant DNA methylation that may contribute to the pathogenesis of acute myeloid leukemia (AML). However, the molecular basis underlying the mutation-induced functional misregulation of DNMT3A remains unclear. Here, we report the crystal structures of the DNMT3A methyltransferase domain, revealing a molecular basis for its oligomerization behavior distinct to DNMT3B, and the enhanced intermolecular contacts caused by the R882H or R882C mutation. Our biochemical, cellular, and genomic DNA methylation analyses demonstrate that introducing the DNMT3B-converting mutations inhibits the R882H-/R882C-triggered DNMT3A polymerization and enhances substrate access, thereby eliminating the dominant-negative effect of the DNMT3A R882 mutations in cells. Together, this study provides mechanistic insights into DNMT3A R882 mutations-triggered aberrant oligomerization and DNA hypomethylation in AML, with important implications in cancer therapy.
Organizational Affiliation:
Department of Biochemistry, University of California, Riverside, CA, USA.