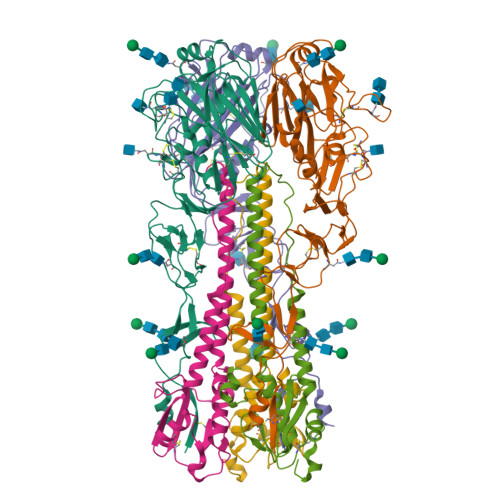
Engineering a cleaved, prefusion-stabilized influenza B virus hemagglutinin by identification and locking of all six pH switches.
Juraszek, J., Milder, F.J., Yu, X., Blokland, S., van Overveld, D., Abeywickrema, P., Tamara, S., Sharma, S., Rutten, L., Bakkers, M.J.G., Langedijk, J.P.M.(2024) PNAS Nexus 3: pgae462-pgae462
- PubMed: 39445049
- DOI: https://doi.org/10.1093/pnasnexus/pgae462
- Primary Citation of Related Structures:
8UAD - PubMed Abstract:
Vaccine components based on viral fusion proteins require high stability of the native prefusion conformation for optimal potency and manufacturability. In the case of influenza B virus hemagglutinin (HA), the stem's conformation relies on efficient cleavage. In this study, we identified six pH-sensitive regions distributed across the entire ectodomain where protonated histidines assume either a repulsive or an attractive role. Substitutions in these areas enhanced the protein's expression, quality, and stability in its prefusion trimeric state. Importantly, this stabilization enabled the production of a cleavable HA0, which is further processed into HA1 and HA2 by furin during exocytic pathway passage, thereby facilitating correct folding, increased stability, and screening for additional stabilizing substitutions in the core of the metastable fusion domain. Cryo-EM analysis at neutral and low pH revealed a previously unnoticed pH switch involving the C-terminal residues of the natively cleaved HA1. This switch keeps the fusion peptide in a clamped state at neutral pH, averting premature conformational shift. Our findings shed light on new strategies for possible improvements of recombinant or genetic-based influenza B vaccines.
Organizational Affiliation:
Janssen Vaccines & Prevention BV, 2333 CN Leiden, The Netherlands.