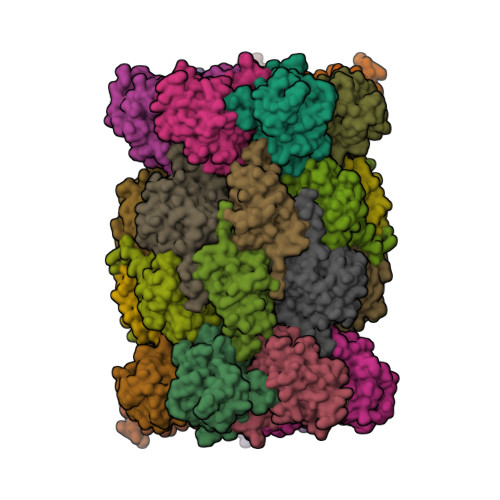
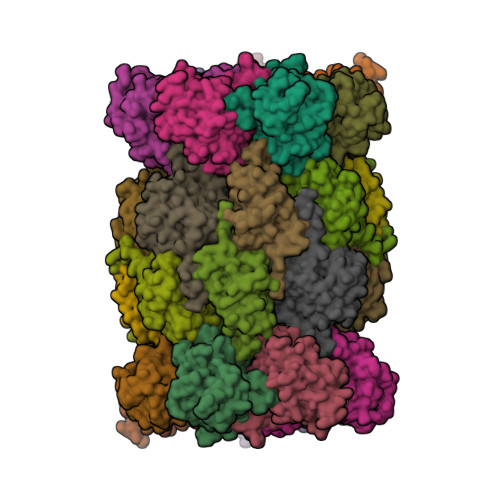
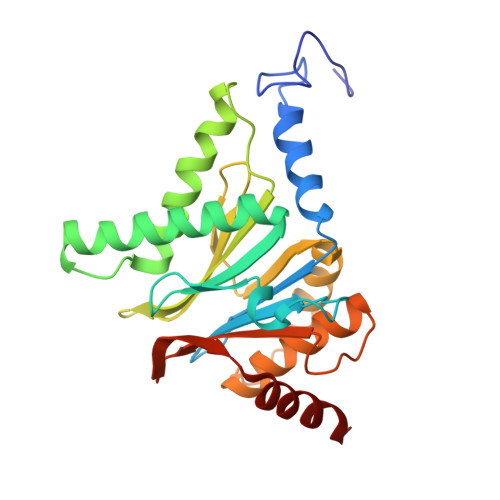
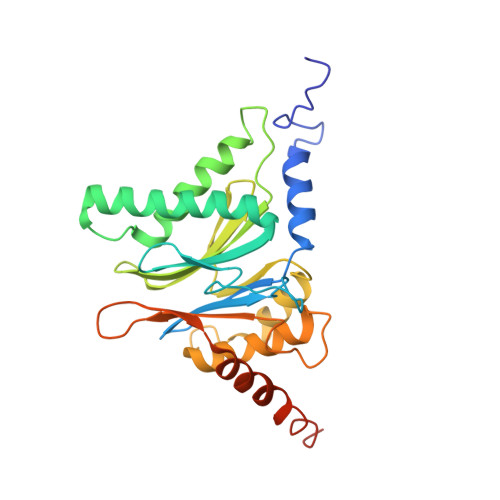
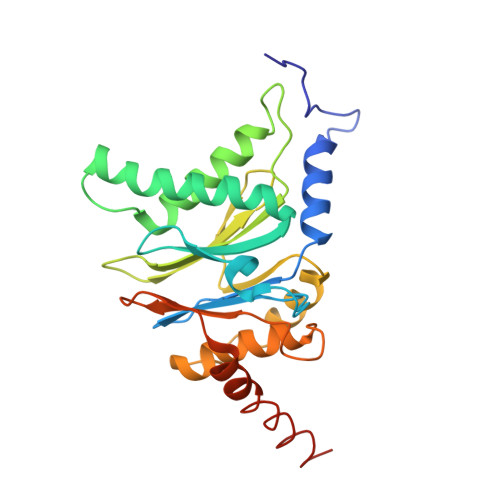
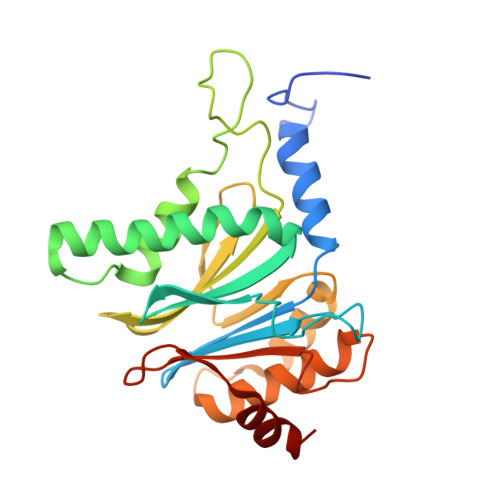
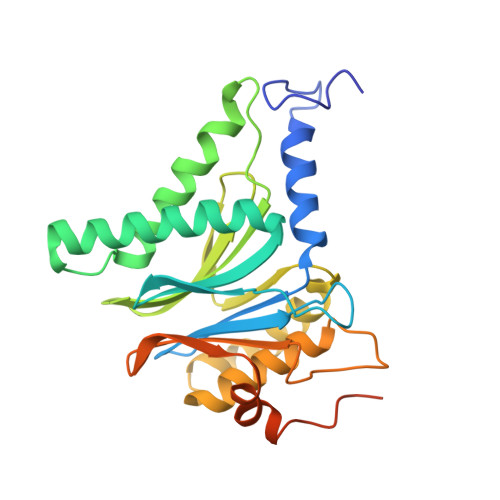
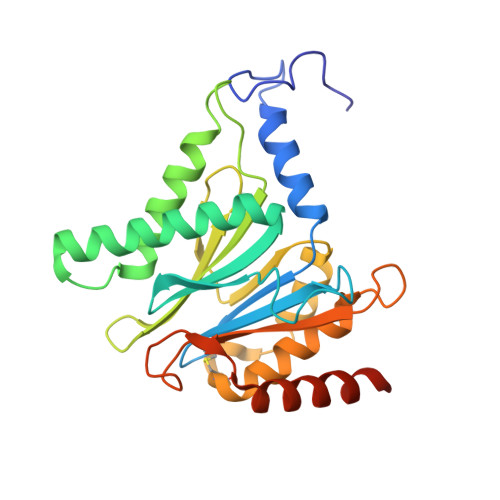
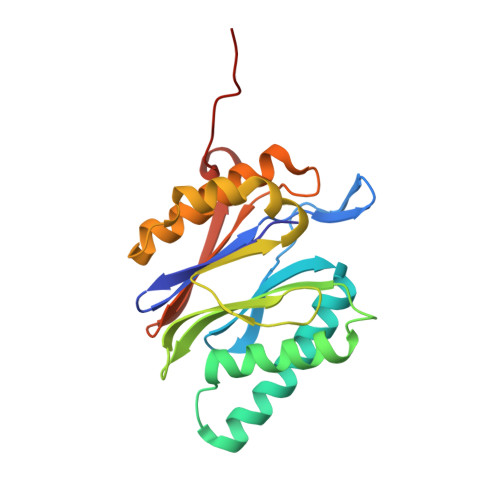
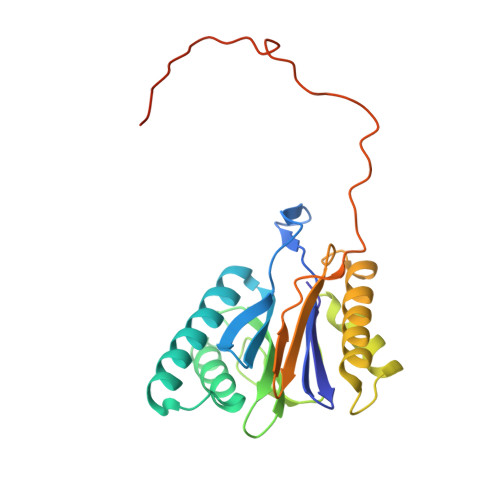
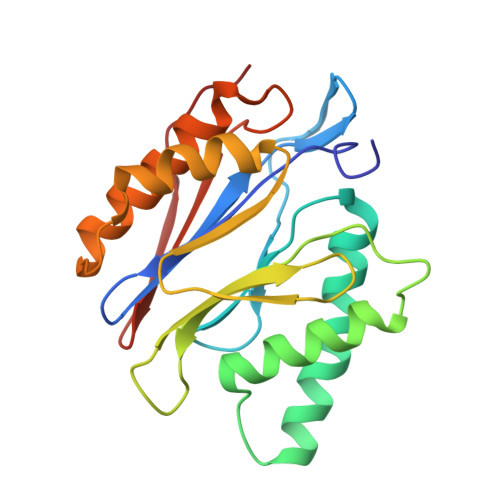
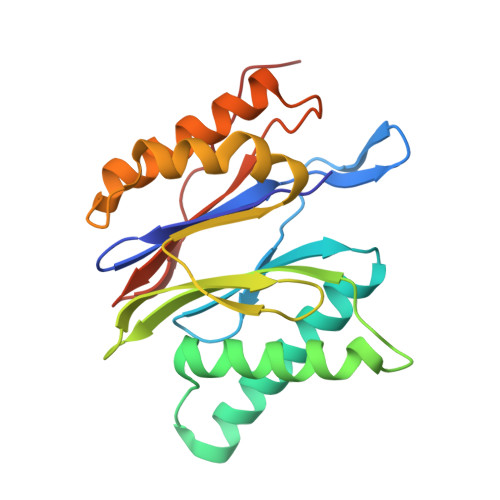
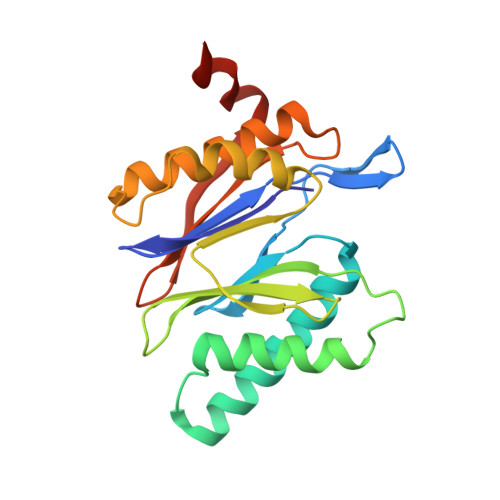
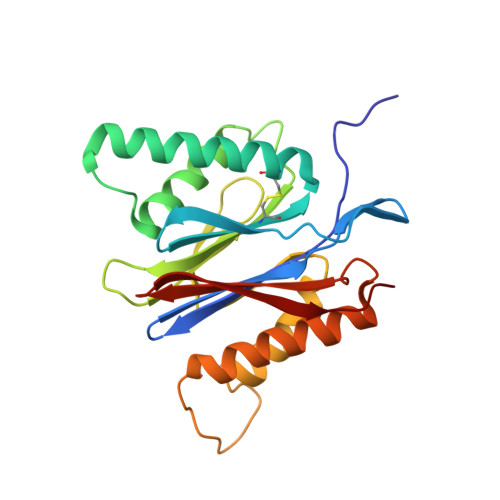
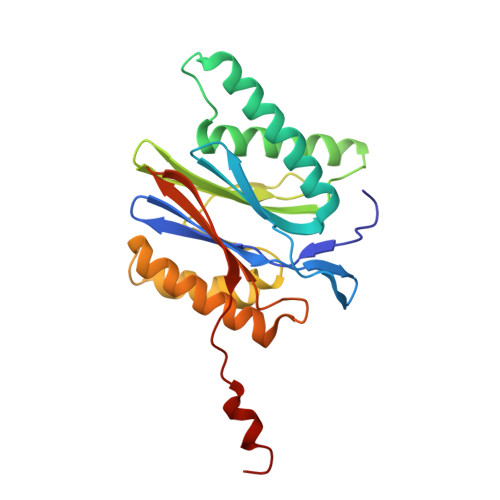
The structure of the mammalian 20S proteasome at 2.75 A resolution.
Unno, M., Mizushima, T., Morimoto, Y., Tomisugi, Y., Tanaka, K., Yasuoka, N., Tsukihara, T.(2002) Structure 10: 609-618
- PubMed: 12015144
- DOI: https://doi.org/10.1016/s0969-2126(02)00748-7
- Primary Citation of Related Structures:
1IRU - PubMed Abstract:
The 20S proteasome is the catalytic portion of the 26S proteasome. Constitutively expressed mammalian 20S proteasomes have three active subunits, beta 1, beta 2, and beta 5, which are replaced in the immunoproteasome by interferon-gamma-inducible subunits beta 1i, beta 2i, and beta 5i, respectively. Here we determined the crystal structure of the bovine 20S proteasome at 2.75 A resolution. The structures of alpha 2, beta 1, beta 5, beta 6, and beta 7 subunits of the bovine enzyme were different from the yeast enzyme but enabled the bovine proteasome to accommodate either the constitutive or the inducible subunits. A novel N-terminal nucleophile hydrolase activity was proposed for the beta 7 subunit. We also determined the site of the nuclear localization signals in the molecule. A model of the immunoproteasome was predicted from this constitutive structure.
Organizational Affiliation:
Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Osaka 565-0871, Japan.