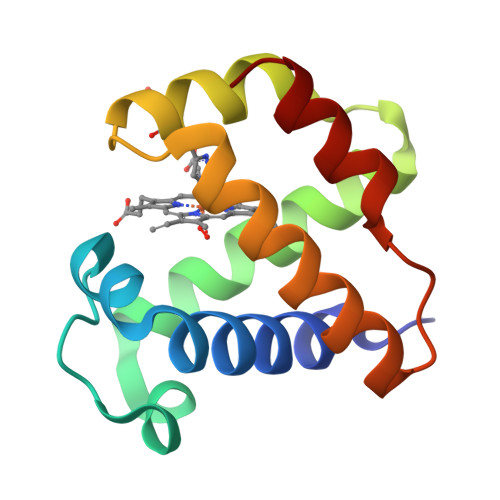
Thr-E11 Regulates O2 Affinity in Cerebratulus Lacteus Mini-Hemoglobin
Pesce, A., Nardini, M., Ascenzi, P., Geuens, E., Dewilde, S., Moens, L., Bolognesi, M., Riggs, A., Hale, A., Deng, P., Nienhaus, G.U., Olson, J.S., Nienhaus, K.(2004) J Biological Chem 279: 33662
- PubMed: 15161908
- DOI: https://doi.org/10.1074/jbc.M403597200
- Primary Citation of Related Structures:
1V07 - PubMed Abstract:
The mini-hemoglobin from Cerebratulus lacteus (CerHb) belongs to a class of globins containing the polar Tyr-B10/Gln-E7 amino acid pair that normally causes low rates of O2 dissociation and ultra-high O2 affinity, which suggest O2 sensing or NO scavenging functions. CerHb, however, has high rates of O2 dissociation (kO2 = 200-600 s(-1)) and moderate O2 affinity (KO2) approximately 1 microm(-1)) as a result of a third polar amino acid in its active site, Thr-E11. When Thr-E11 is replaced by Val, kO2 decreases 1000-fold and KO2 increases 130-fold at pH 7.0, 20 degrees C. The mutation also shifts the stretching frequencies of both heme-bound and photodissociated CO, indicating marked changes of the electrostatic field at the active site. The crystal structure of Thr-E11 --> Val CerHbO2 at 1.70 A resolution is almost identical to that of the wild-type protein (root mean square deviation of 0.12 A). The dramatic functional and spectral effects of the Thr-E11 --> Val mutation are due exclusively to changes in the hydrogen bonding network in the active site. Replacing Thr-E11 with Val "frees" the Tyr-B10 hydroxyl group to rotate toward and donate a strong hydrogen bond to the heme-bound ligand, causing a selective increase in O2 affinity, a decrease of the rate coefficient for O2 dissociation, a 40 cm(-1) decrease in nuCO of heme-bound CO, and an increase in ligand migration toward more remote intermediate sites.
Organizational Affiliation:
Department of Physics-INFM and Center for Excellence in Biomedical Research, University of Genova, Via Dodecaneso 33, 16146 Genova, Italy.