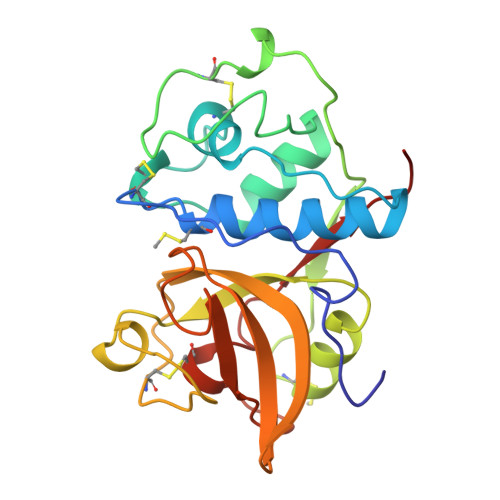
Structure of chymopapain at 1.7 A resolution.
Maes, D., Bouckaert, J., Poortmans, F., Wyns, L., Looze, Y.(1996) Biochemistry 35: 16292-16298
- PubMed: 8973203
- DOI: https://doi.org/10.1021/bi961491w
- Primary Citation of Related Structures:
1YAL - PubMed Abstract:
The X-ray structure of chymopapain, a cysteine proteinase isolated from the latex of the fruits of Carica papaya L., has been determined by molecular replacement methods and refined to a conventional R factor of 0.19 for all observed reflections in the range from 9.5 to 1.7 A resolution. The crystals used in this study contained a unique molecular species of chymopapain with two moles of thiomethyl attached to the two free cysteines per mole of enzyme. A comparison is made with the other known papaya proteinase X-ray structures: papain, caricain, and glycyl endopeptidase. Their backbone conformations are extremely similar except for two loop regions. Both regions are located at the surface of the protein and far away of the active site cleft. In each X-ray structure the same water network was found at the interface between the two domains of the enzyme. A close examination of the active site groove showed that the specificity restrictions dictated by the S2 subsite did not differ significantly among the four proteinases.
Organizational Affiliation:
Ultrastructure Unit, Vlaams Interuniversitair Instituut voor Biotechnologie, Vrije Universiteit Brussel, Belgium.