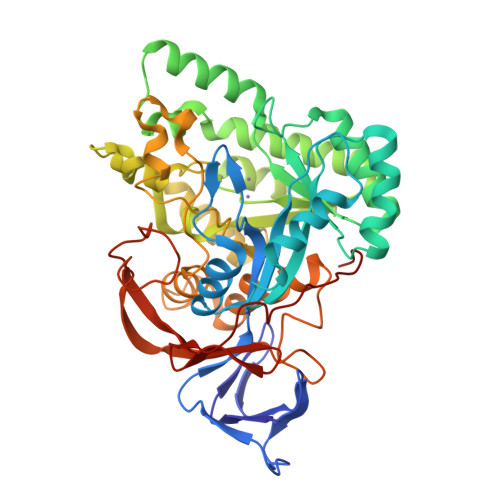
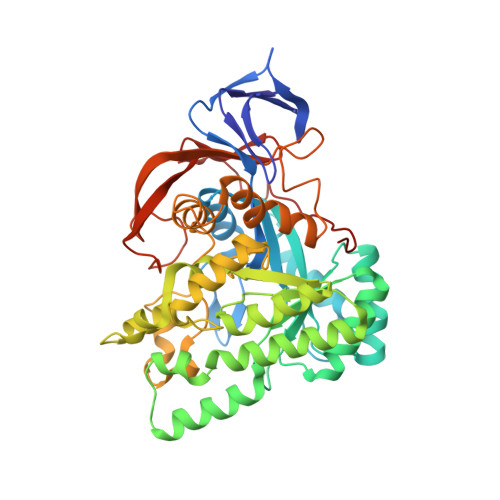
Molecular structure of D-hydantoinase from Bacillus sp. AR9: evidence for mercury inhibition.
Radha Kishan, K.V., Vohra, R.M., Ganesan, K., Agrawal, V., Sharma, V.M., Sharma, R.(2005) J Mol Biology 347: 95-105
- PubMed: 15733920
- DOI: https://doi.org/10.1016/j.jmb.2005.01.025
- Primary Citation of Related Structures:
1YNY - PubMed Abstract:
Stereospecific conversion of hydantoins into their carbamoyl acid derivatives could be achieved by using the enzyme hydantoinase. Specific hydantoinases convert either the D-form or the L-form of the hydantoin and the amino acids responsible for stereospecificity have not been identified. Structural studies on hydantoinases from a few bacterial species were published recently. The structure of a thermostable D-hydantoinase from Bacillus sp. AR9 (bar9HYD) was solved to 2.3 angstroms resolution. The usual modification of carboxylation of the active-site residue Lys150 did not happen in bar9HYD. Two manganese ions were modelled in the active site. Through biochemical studies, it was shown that mercury inhibits the activity of the enzyme. The mercury derivative provided some information about the binding site of the mercuric inhibitors and a possible reason for inhibition is presented.
Organizational Affiliation:
Institute of Microbial Technology, Sector 39-A, Chandigarh 160 036, India. kishan@imtech.res.in