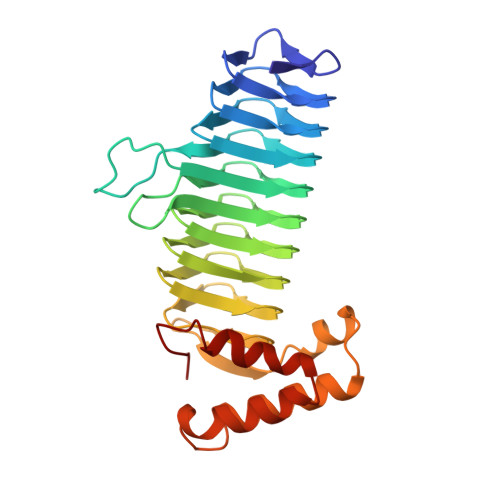
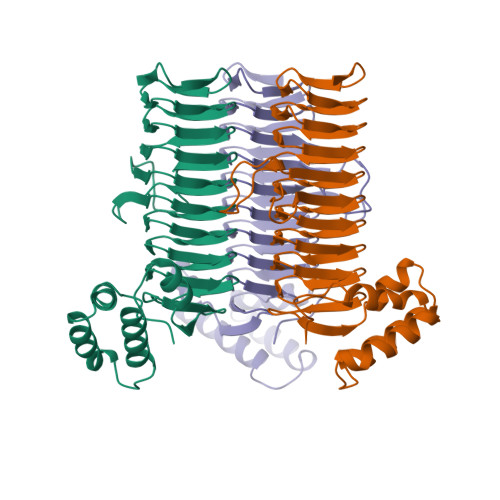
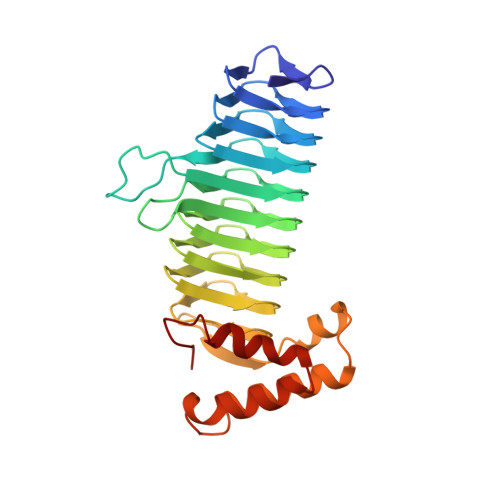
A left-handed parallel beta helix in the structure of UDP-N-acetylglucosamine acyltransferase.
Raetz, C.R., Roderick, S.L.(1995) Science 270: 997-1000
- PubMed: 7481807
- DOI: https://doi.org/10.1126/science.270.5238.997
- Primary Citation of Related Structures:
1LXA - PubMed Abstract:
UDP-N-acetylglucosamine 3-O-acyltransferase (LpxA) catalyzes the transfer of (R)-3-hydroxymyristic acid from its acyl carrier protein thioester to UDP-N-acetylglucosamine. LpxA is the first enzyme in the lipid A biosynthetic pathway and is a target for the design of antibiotics. The x-ray crystal structure of LpxA has been determined to 2.6 angstrom resolution and reveals a domain motif composed of parallel beta strands, termed a left-handed parallel beta helix (L beta H). This unusual fold displays repeated violations of the protein folding constraint requiring right-handed crossover connections between strands of parallel beta sheets and may be present in other enzymes that share amino acid sequence homology to the repeated hexapeptide motif of LpxA.
Organizational Affiliation:
Department of Biochemistry, Duke University Medical Center, Durham, NC 22710, USA.