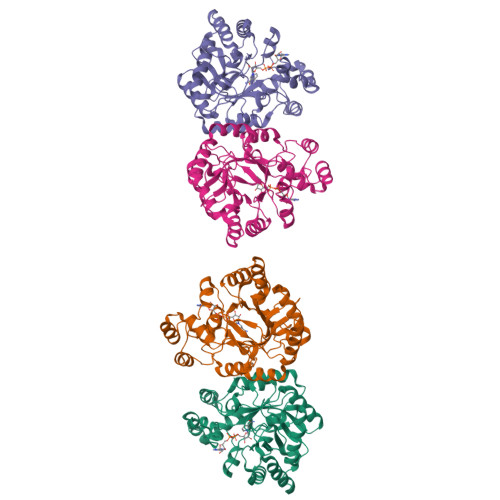
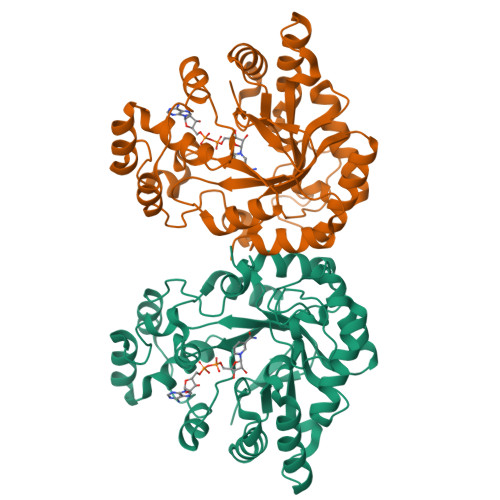
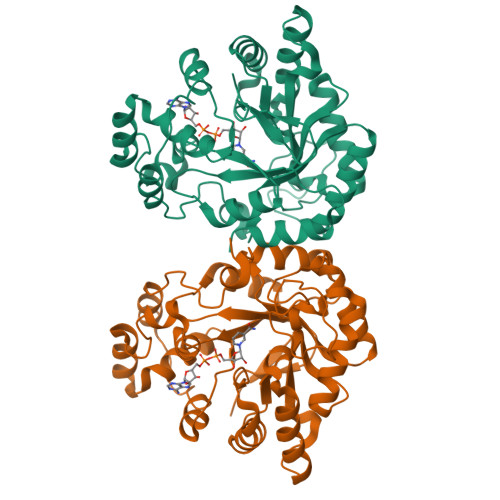
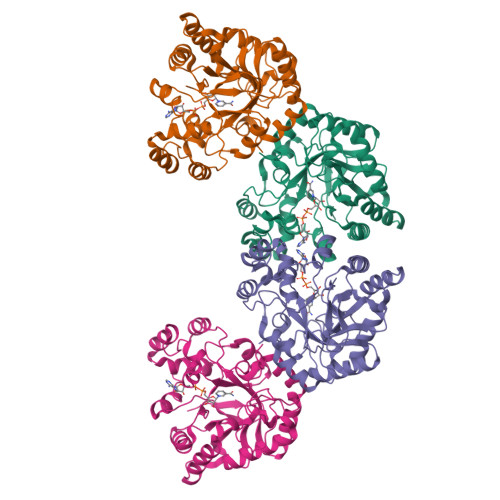
Structure of xylose reductase bound to NAD+ and the basis for single and dual co-substrate specificity in family 2 aldo-keto reductases
Kavanagh, K.L., Klimacek, M., Nidetzky, B., Wilson, D.K.(2003) Biochem J 373: 319-326
- PubMed: 12733986
- DOI: https://doi.org/10.1042/BJ20030286
- Primary Citation of Related Structures:
1MI3 - PubMed Abstract:
The co-ordinates reported have been submitted to the Protein Data Bank under accession number 1MI3. Xylose reductase (XR; AKR2B5) is an unusual member of aldo-keto reductase superfamily, because it is one of the few able to efficiently utilize both NADPH and NADH as co-substrates in converting xylose into xylitol. In order to better understand the basis for this dual specificity, we have determined the crystal structure of XR from the yeast Candida tenuis in complex with NAD(+) to 1.80 A resolution (where 1 A=0.1 nm) with a crystallographic R -factor of 18.3%. A comparison of the NAD(+)- and the previously determined NADP(+)-bound forms of XR reveals that XR has the ability to change the conformation of two loops. To accommodate both the presence and absence of the 2'-phosphate, the enzyme is able to adopt different conformations for several different side chains on these loops, including Asn(276), which makes alternative hydrogen-bonding interactions with the adenosine ribose. Also critical is the presence of Glu(227) on a short rigid helix, which makes hydrogen bonds to both the 2'- and 3'-hydroxy groups of the adenosine ribose. In addition to changes in hydrogen-bonding of the adenosine, the ribose unmistakably adopts a 3'- endo conformation rather than the 2'- endo conformation seen in the NADP(+)-bound form. These results underscore the importance of tight adenosine binding for efficient use of either NADH or NADPH as a co-substrate in aldo-keto reductases. The dual specificity found in XR is also an important consideration in designing a high-flux xylose metabolic pathway, which may be improved with an enzyme specific for NADH.
Organizational Affiliation:
Section of Molecular and Cellular Biology, University of California, Davis, CA 95616, USA.