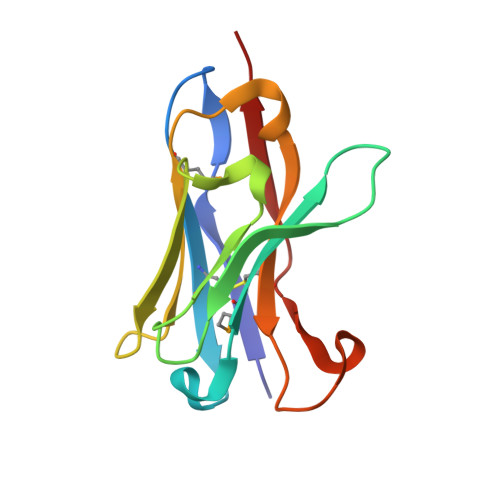
Crystal Structure of a Human Vh: Requirements for Maintaining a Monomeric Fragment
Dottorini, T., Vaughan, C.K., Walsh, M.A., Losurdo, P., Sollazzo, M.(2004) Biochemistry 43: 622
- PubMed: 14730966
- DOI: https://doi.org/10.1021/bi035800b
- Primary Citation of Related Structures:
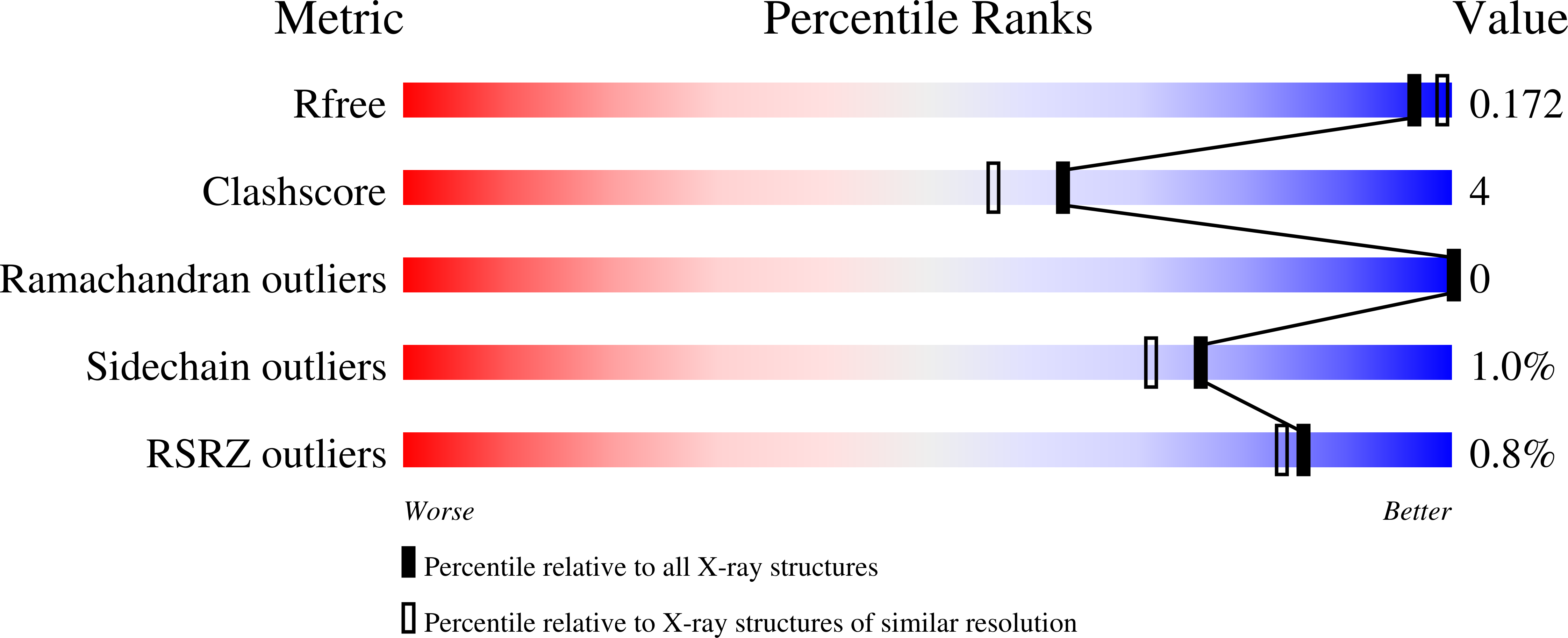
1OL0 - PubMed Abstract:
The variable domain of dromedary immunoglobulins comprises only the heavy chain and is missing the light-chain variable domain. This single domain is sufficient for antigen recognition and binding-half that required by other mammals. Human antibody-VHs have previously been camelized to be soluble stable fragments that retain antigen binding. Such engineered VHH are of interest in drug development, since they are nonimmunogenic, and in other biotechnology applications. We present the structure of a camelized human antibody fragment (cVH), which is a competitive and reversible inhibitor of the NS3 serine protease of the hepatitis C virus (HCV). In solution, this cVH undergoes a concentration-dependent monomer-dimer equilibrium. The structure confirms the minimum mutational requirements of the VL-binding face. The fragment also suggests a means by which the observed dimerization occurs, highlighting the importance of the composition of the CDR3 in maintaining a truly camelized VH.
Organizational Affiliation:
Istituto di Ricerche di Biologia Molecolare, Crystallography Unit, Via Pontina km 30600, 00040 Pomezia (Rm), Italy.