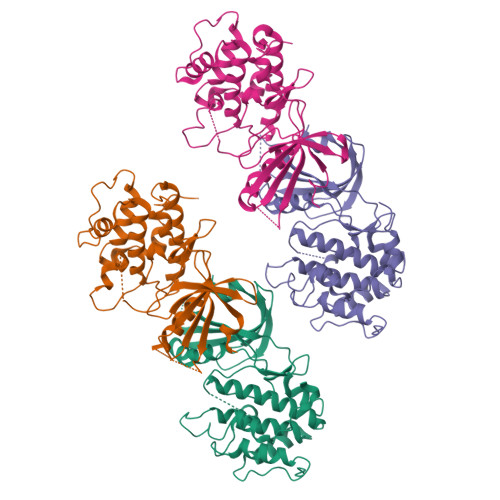
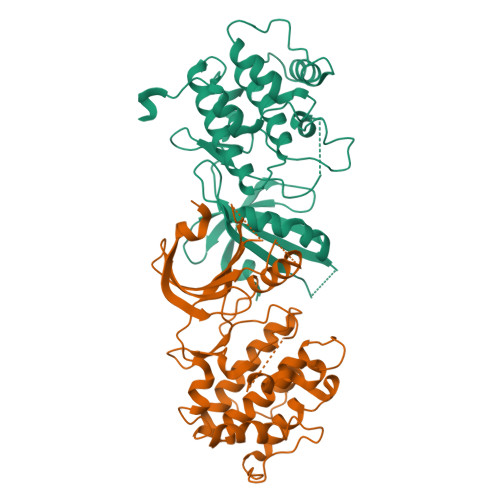
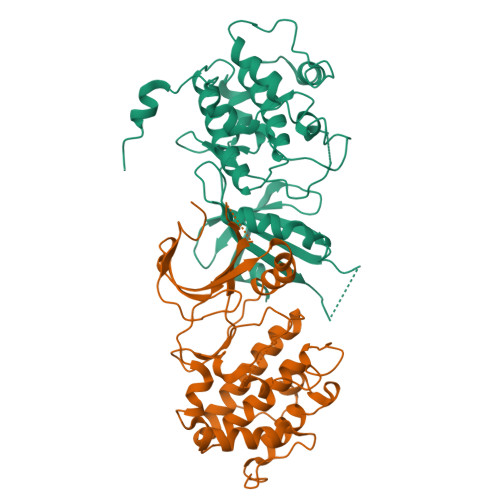
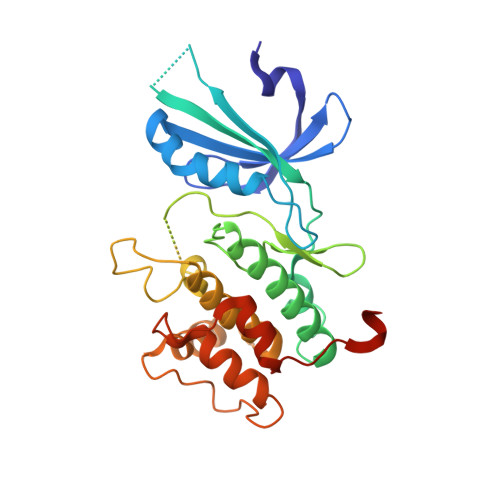
Structural Basis for Autoinhibition and Mutational Activation of Eukaryotic Initiation Factor 2{alpha} Protein Kinase GCN2
Padyana, A.K., Qiu, H., Roll-Mecak, A., Hinnebusch, A.G., Burley, S.K.(2005) J Biol Chem 280: 29289-29299
- PubMed: 15964839
- DOI: https://doi.org/10.1074/jbc.M504096200
- Primary Citation of Related Structures:
1ZXE, 1ZY4, 1ZY5, 1ZYC, 1ZYD - PubMed Abstract:
The GCN2 protein kinase coordinates protein synthesis with levels of amino acid stores by phosphorylating eukaryotic translation initiation factor 2. The autoinhibited form of GCN2 is activated in cells starved of amino acids by binding of uncharged tRNA to a histidyl-tRNA synthetase-like domain. Replacement of Arg-794 with Gly in the PK domain (R794G) activates GCN2 independently of tRNA binding. Crystal structures of the GCN2 protein kinase domain have been determined for wild-type and R794G mutant forms in the apo state and bound to ATP/AMPPNP. These structures reveal that GCN2 autoinhibition results from stabilization of a closed conformation that restricts ATP binding. The R794G mutant shows increased flexibility in the hinge region connecting the N- and C-lobes, resulting from loss of multiple interactions involving Arg794. This conformational change is associated with intradomain movement that enhances ATP binding and hydrolysis. We propose that intramolecular interactions following tRNA binding remodel the hinge region in a manner similar to the mechanism of enzyme activation elicited by the R794G mutation.
Organizational Affiliation:
Structural GenomiX, Inc., San Diego, CA 92121, USA.