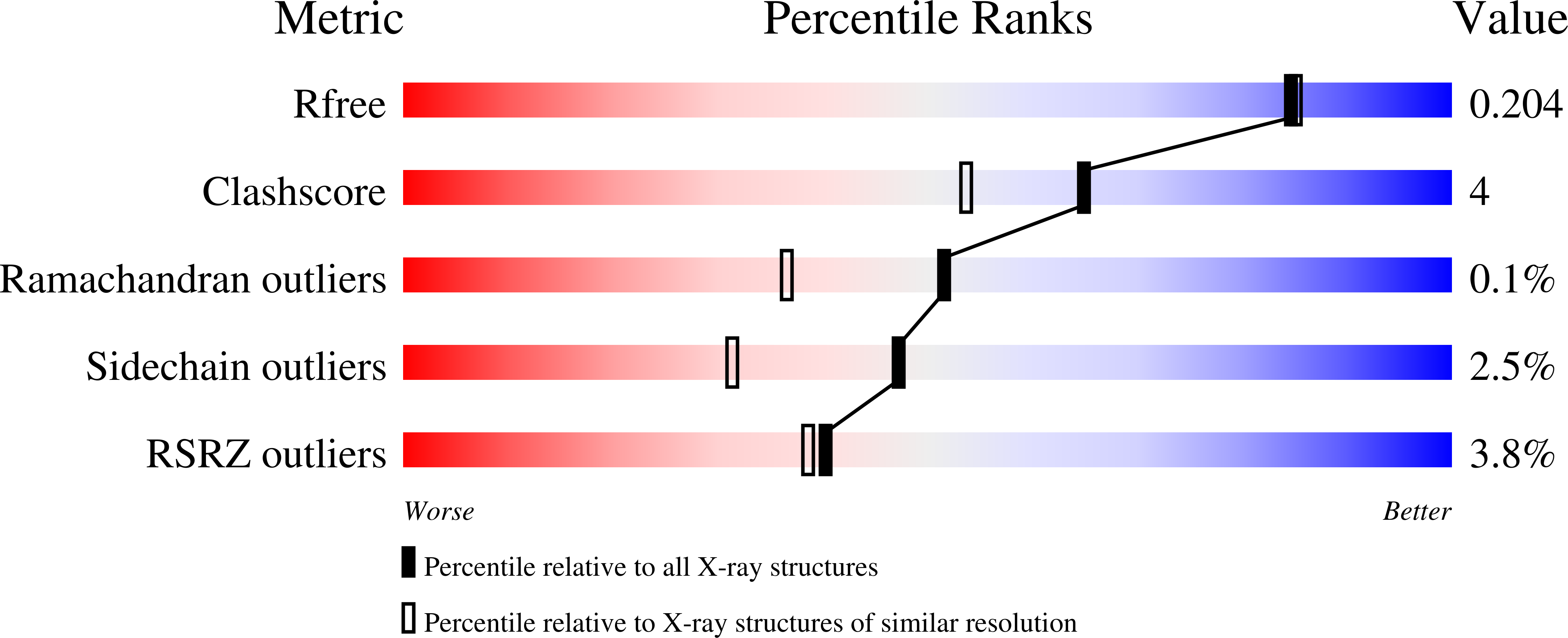
Exploring the Binding Conformations of Bulkier Dipeptide Amide Inhibitors in Constitutive Nitric Oxide Synthases.
Li, H., Flinspach, M.L., Igarashi, J., Jamal, J., Yang, W., Litzinger, E.A., Huang, H., Erdal, E.P., Silverman, R.B., Poulos, T.L.(2005) Biochemistry 44: 15222-15229
- PubMed: 16285725
- DOI: https://doi.org/10.1021/bi0513610
- Primary Citation of Related Structures:
1ZZQ, 1ZZR, 1ZZS, 1ZZT, 1ZZU - PubMed Abstract:
A series of L-nitroarginine-based dipeptide inhibitors are highly selective for neuronal nitric oxide synthase (nNOS) over the endothelial isoform (eNOS). Crystal structures of these dipeptides bound to both isoforms revealed two different conformations, curled in nNOS and extended in eNOS, corresponding to higher and lower binding affinity to the two isoforms, respectively. In previous studies we found that the primary reason for selectivity is that Asp597 in nNOS, which is Asn368 in eNOS, provides greater electrostatic stabilization in the inhibitor complex. While this is the case for smaller dipeptide inhibitors, electrostatic stabilization may no longer be the sole determinant for isoform selectivity with bulkier dipeptide inhibitors. Another residue farther away from the active site, Met336 in nNOS (Val106 in eNOS), is in contact with bulkier dipeptide inhibitors. Double mutants were made to exchange the D597/M336 pair in nNOS with N368/V106 in eNOS. Here we report crystal structures and inhibition constants for bulkier dipeptide inhibitors bound to nNOS and eNOS that illustrate the important role played by residues near the entry to the active site in isoform selective inhibition.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, University of California, Irvine, California 92697-3900, USA.