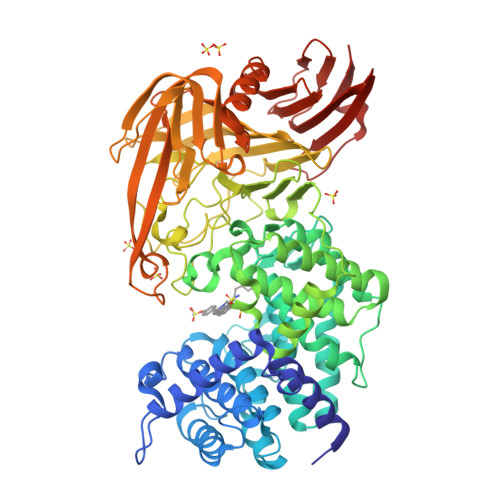
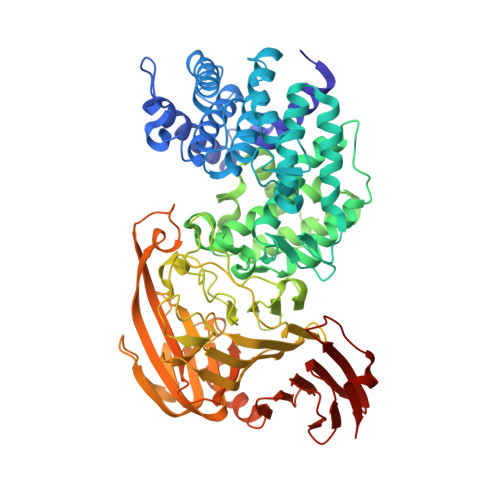
Design of new benzoxazole-2-thione-derived inhibitors of Streptococcus pneumoniae hyaluronan lyase: structure of a complex with a 2-phenylindole.
Rigden, D.J., Botzki, A., Nukui, M., Mewbourne, R.B., Lamani, E., Braun, S., von Angerer, E., Bernhardt, G., Dove, S., Buschauer, A., Jedrzejas, M.J.(2006) Glycobiology 16: 757-765
- PubMed: 16638841
- DOI: https://doi.org/10.1093/glycob/cwj116
- Primary Citation of Related Structures:
2BRP - PubMed Abstract:
The bacterial hyaluronan lyases (Hyals) that degrade hyaluronan, an important component of the extracellular matrix, are involved in microbial spread. Inhibitors of these enzymes are essential in investigation of the role of hyaluronan and Hyal in bacterial infections and constitute a new class of antibiotics against Hyal-producing bacteria. Recently, we identified 1,3-diacetylbenzimidazole-2-thione and related molecules as inhibitors of streptococcal Hyal. One of such compounds, 1-decyl-2-(4-sulfamoyloxyphenyl)-1-indol-6-yl sulfamate, was co-crystallized in a complex with Streptococcus pneumoniae Hyal and its structure elucidated. The resultant X-ray structure demonstrates that this inhibitor fits in the enzymatic active site via interactions resembling the binding mode of the natural hyaluronan substrate. X-ray structural analysis also indicates binding interactions with the catalytic residues and those of a catalytically essential hydrophobic patch. An IC50 value of 11 microM for Hyal from Streptococcus agalactiae (strain 4755) qualifies this phenylindole compound as one of the most potent Hyal inhibitors known to date. The structural data suggested a similar binding mode for N-(3-phenylpropionyl)-benzoxazole-2-thione. This new compound's inhibitory properties were confirmed resulting in discovery of yet another Hyal inhibitor (IC50 of 15 microM). These benzoxazole-2-thiones constitute a new class of inhibitors of bacterial Hyals and are well suited for further optimization of their selectivity, potency, and pharmacokinetic properties.
Organizational Affiliation:
Institute of Pharmacy, University of Regensburg, 93040 Regensburg, Germany.