Structural and mechanistic studies of the orf12 gene product from the clavulanic acid biosynthesis pathway.
Valegard, K., Iqbal, A., Kershaw, N.J., Ivison, D., Genereux, C., Dubus, A., Blikstad, C., Demetriades, M., Hopkinson, R.J., Lloyd, A.J., Roper, D.I., Schofield, C.J., Andersson, I., McDonough, M.A.(2013) Acta Crystallogr D Biol Crystallogr 69: 1567-1579
- PubMed: 23897479
- DOI: https://doi.org/10.1107/S0907444913011013
- Primary Citation of Related Structures:
2XEP, 2XF3, 2XFS, 2XFT, 2XGN, 2XH9 - PubMed Abstract:
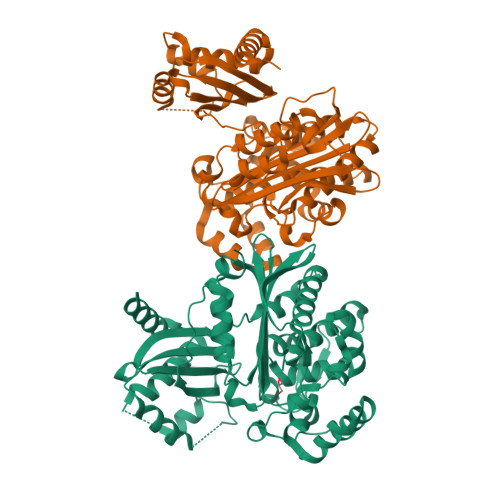
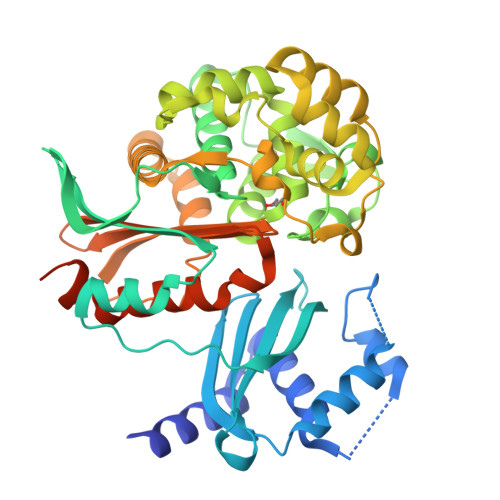
Structural and biochemical studies of the orf12 gene product (ORF12) from the clavulanic acid (CA) biosynthesis gene cluster are described. Sequence and crystallographic analyses reveal two domains: a C-terminal penicillin-binding protein (PBP)/β-lactamase-type fold with highest structural similarity to the class A β-lactamases fused to an N-terminal domain with a fold similar to steroid isomerases and polyketide cyclases. The C-terminal domain of ORF12 did not show β-lactamase or PBP activity for the substrates tested, but did show low-level esterase activity towards 3'-O-acetyl cephalosporins and a thioester substrate. Mutagenesis studies imply that Ser173, which is present in a conserved SXXK motif, acts as a nucleophile in catalysis, consistent with studies of related esterases, β-lactamases and D-Ala carboxypeptidases. Structures of wild-type ORF12 and of catalytic residue variants were obtained in complex with and in the absence of clavulanic acid. The role of ORF12 in clavulanic acid biosynthesis is unknown, but it may be involved in the epimerization of (3S,5S)-clavaminic acid to (3R,5R)-clavulanic acid.
Organizational Affiliation:
Department of Molecular Biology, Swedish University of Agricultural Sciences, Box 590, S-751 24 Uppsala, Sweden.